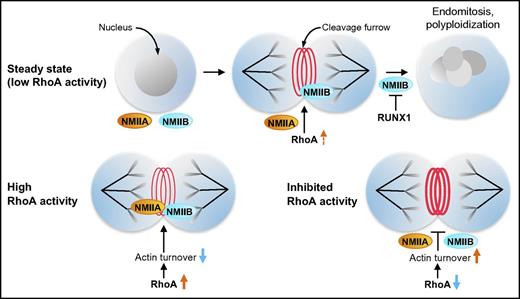
Megakaryocytes express 2 NMII isoforms, NMIIA and NMIIB. At steady state, low RhoA/ROCK activity results in the localization of NMIIB, but not NMIIA, at the megakaryocyte cleavage furrow, a step required for the initiation of endomitosis. Subsequent downregulation of NMIIB expression by RUNX1 facilitates progression of endomitosis and polyploidization. Increasing RhoA activity and, subsequently, decreasing actin turnover in megakaryocytes results in the localization of both NMIIB and NMIIA at the cleavage furrow. Conversely, further inhibition of basal low RhoA activity increases actin turnover and leads to the loss of NMIIB from the cleavage furrow.
Megakaryocytes express 2 NMII isoforms, NMIIA and NMIIB. At steady state, low RhoA/ROCK activity results in the localization of NMIIB, but not NMIIA, at the megakaryocyte cleavage furrow, a step required for the initiation of endomitosis. Subsequent downregulation of NMIIB expression by RUNX1 facilitates progression of endomitosis and polyploidization. Increasing RhoA activity and, subsequently, decreasing actin turnover in megakaryocytes results in the localization of both NMIIB and NMIIA at the cleavage furrow. Conversely, further inhibition of basal low RhoA activity increases actin turnover and leads to the loss of NMIIB from the cleavage furrow.
Megakaryocyte endomitosis is a tightly regulated process that is only incompletely understood at the molecular level.2 Endomitosis, which requires cell-cycle regulators such as cyclin B and cyclin B–dependent Cdc2 kinase, begins after an initial phase of mitotic replication and leads to an increase in DNA content to up to 128N in a single megakaryocyte nucleus.2 Cytokinesis failure was shown to result from a defect in formation of the cleavage furrow, a contractile ring composed of nonmuscle NMII and actin filaments.3
Megakaryocytes express 2 NMII isoforms, NMIIA and NMIIB. Mutations in MYH9, the gene encoding NMIIA, give rise to platelet disorders characterized by macrothrombocytopenia and variable bleeding that are summarized by the term MYH9-related disorders (MYH9-RD). It has been shown that NMIIA expression increases during megakaryocyte maturation, whereas that of NMIIB decreases. Exclusively, NMIIB localizes at the cleavage furrow of mitotic (2N-4N) megakaryocytes, and downregulation of its gene (MYH10) by the transcription factor RUNX1 is critical for the switch from mitosis to endomitosis.4
Several studies point to a critical role of the small GTPase RhoA and its downstream effector ROCK in inducing the contraction force necessary to complete cytokinesis. Focusing on the RhoA/ROCK pathway, in the current study, Roy et al aimed to elucidate the mechanism leading to specific NMIIB, but not NMIIA, localization at the cleavage furrow in human megakaryocytes. They showed that under conditions of low RhoA activity, NMIIB was the predominant active form and, as previously reported,4 the only one localizing at the megakaryocyte cleavage furrow (see figure). In contrast, erythroblasts, which do not polyploidize, showed higher basal RhoA activity, and both NMIIA and NMIIB localized at the cleavage furrow, indicating that RhoA/ROCK signaling dose-dependently regulates NMII isoform activation and differential localization. Indeed, inhibition of basal RhoA/ROCK signaling in megakaryocytes abolished localization of NMIIB at the cleavage furrow, whereas an identical treatment of erythroblasts only resulted in loss of NMIIA, but not NMIIB, from the furrow. Consistently, expression of constitutively active RhoAL63 in megakaryocytes induced the localization of both NMIIA and NMIIB isoforms at the cleavage furrow (see figure). Because classical RhoA/ROCK signaling primarily affects the actin cytoskeleton, the authors proceeded to study this pathway and found a higher actin turnover at steady state in megakaryocytes compared with erythroblasts. Strikingly, treatment of erythroblasts with actin-depolymerizing agents resulted in loss of NMIIA, but not NMIIB, from the cleavage furrow, and the same treatment did not affect NMIIB localization in megakaryocytes. Thus, RhoA/ROCK-dependent regulation of actin turnover appears to be sufficient to induce differential localization of NMIIA and NMIIB at the cleavage furrow. Notably, the effect of RhoA/ROCK signaling on NMII isoform localization was independent of myosin light chain (MLC) phosphorylation, suggesting that the downstream effects exerted from altered actin turnover are at least in part independent from those mediated by MLC phosphorylation.
Taken together, the result from Roy et al strongly suggests that megakaryocyte cytokinesis failure occurs as a direct consequence of continuous inhibition of NMIIA at the cleavage furrow by low RhoA/ROCK signaling and increased actin turnover (see figure). The subsequent downregulation of the gene encoding NMIIB, namely MYH10, by RUNX1 is required for the propagation of endomitosis.
These results are in agreement with previous reports showing that inhibition of the RhoA/ROCK pathway led to decreased F-actin at the cleavage furrow while increasing ploidy.5 Increased ploidy was also observed in megakaryocytes derived from conditional (Pf4cre) RhoA knockout mice.6 The authors’ finding that 2 other prominent GTPases in platelets, namely Rac1 and Cdc42, are not involved in regulation of NMII isoform localization and endomitosis is in line with results from mice lacking both GTPases in megakaryocytes.7
Despite focusing on the cleavage furrow, the authors observed a similar impact of RhoA/ROCK signaling on actin turnover and NMII isoform localization at the cell cortex. This is noteworthy because several previous studies suggest that the RhoA/ROCK/NMIIA pathway negatively regulates proplatelet formation.2 In addition, ROCK inhibition of megakaryocytes derived from MYH9-RD patients resulted in a decrease in F-actin and improved proplatelet formation, emphasizing a direct significant impact of RhoA-mediated NMIIA activity and localization on platelet production.8 Interestingly, inhibition of ROCK by Fasudil, a clinically used ROCK inhibitor, could restore platelet counts in mice lacking proteasome activity in megakaryocytes associated with hyperactive RhoA.9 Whether NMII isoforms are downstream effectors of RhoA in this physiologic setting remains to be investigated.
The apparent positive impact of RhoA/ROCK inhibition on platelet production stands in clear contrast to the finding that mice lacking RhoA in megakaryocytes exhibit pronounced macrothrombocytopenia despite only moderately decreased platelet life span in vivo and functional proplatelet formation in vitro.6,10 This discrepancy clearly indicates that RhoA deficiency in vivo affects platelet biogenesis in a way that cannot be reflected using cell culture models.
Together, the results of the study by Roy and colleagues emphasize the complexity and continuous fine-tuning of RhoA/ROCK signaling in megakaryocytes. Further studies will be required to dissect the molecular mechanisms of RhoA signaling in megakaryocytes, including those originating from altered actin turnover that specifically affect the subcellular localization of NMII isoforms.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal