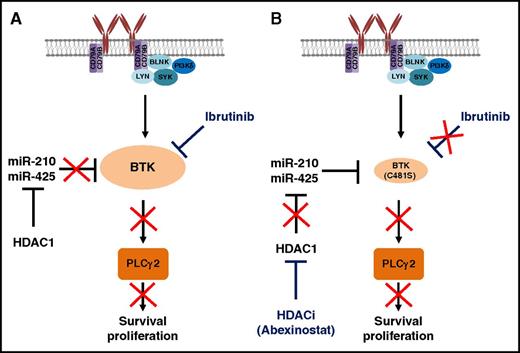
Pharmacological inhibition of HDAC relieves epigenetic suppression of BTK-targeting microRNA. (A) HDAC repressor complex inhibits the function of miR-210 and miR-425, causing an upregulation of BTK. BCR ligation induces activation of BTK, promoting CLL cell survival and proliferation. Ibrutinib covalently binds C481 on BTK to inhibit BTK activity and hence inhibit survival and proliferation of CLL cells. (B) Mutation of BTK C481S prevents covalent binding of ibrutinib to BTK and inhibits ibrutinib activity. The HDACi abexinostat releases HDAC1-mediated suppression of miR-210 and miR-425, leading to a decrease in BTK protein expression and suppression of downstream signaling, even in the presence of C481S mutation. Drugs are highlighted in blue and red crosses indicate the pathway is inhibited. BLNK, B-cell linker; LYN, tyrosine-protein kinase Lyn; SYK, spleen tyrosine kinase.
Pharmacological inhibition of HDAC relieves epigenetic suppression of BTK-targeting microRNA. (A) HDAC repressor complex inhibits the function of miR-210 and miR-425, causing an upregulation of BTK. BCR ligation induces activation of BTK, promoting CLL cell survival and proliferation. Ibrutinib covalently binds C481 on BTK to inhibit BTK activity and hence inhibit survival and proliferation of CLL cells. (B) Mutation of BTK C481S prevents covalent binding of ibrutinib to BTK and inhibits ibrutinib activity. The HDACi abexinostat releases HDAC1-mediated suppression of miR-210 and miR-425, leading to a decrease in BTK protein expression and suppression of downstream signaling, even in the presence of C481S mutation. Drugs are highlighted in blue and red crosses indicate the pathway is inhibited. BLNK, B-cell linker; LYN, tyrosine-protein kinase Lyn; SYK, spleen tyrosine kinase.
B-cell receptor (BCR) signaling is instrumental in the biology of CLL, and kinase inhibitors (KIs) such as ibrutinib and idelalisib, which target BTK and phosphatidylinositol 3-kinase δ (PI3Kδ), respectively, are revolutionizing treatment of this disease. However, these agents are not curative, are taken indefinitely, and resistance and intolerance to these agents is already emerging. Ibrutinib discontinuation is associated with extremely poor survival and represents an important unmet clinical need. The primary resistance mechanism for ibrutinib is through mutations in BTK (C481S) or phospholipase Cγ2 (PLCγ2; R665W and L845F),2 however, microenvironmental signals may also influence sensitivity to this drug.3
miRNAs are small double-stranded RNA molecules of ∼19 to 23 nucleotides, identified as a mechanism that cells use to regulate posttranscriptional expression of various genes and subsequently protein production. They do this by reducing messenger RNA (mRNA) stability, blocking mRNA translation, or promoting mRNA degradation,4 and numerous publications have described important roles for miRNA in CLL pathogenesis and BCR signaling.5,6 HDACs regulate deacetylation and demethylation of lysine residues on histones resulting in the silencing of various genes. In CLL, HDACs are known to inhibit expression of miRNA, particularly miR15a, miR16, and miR29b, which are associated with the suppression of Bcl-2 and Mcl-1.7 However, CLL samples treated in vitro with HDAC inhibitors (HDACi’s) upregulated these miRNAs and consequently suppressed Mcl-1 and Bcl-2 protein expression.7
In this issue, Bottoni et al hypothesized that treatment of CLL cells with HDACi’s may also regulate miRNA that target BTK. Indeed, they demonstrated that BTK protein expression was targeted and reduced most strongly by miR-210 and miR-425 in CLL, and that miR-210 and miR-425 were expressed at lower levels in primary CLL samples compared with normal B cells. They suggested this reduced expression in CLL cells was caused by silencing of BTK-targeting miRNAs via the HDAC repressor complex, indicating a novel outcome of epigenetic silencing, as well as providing a potential explanation for the overexpression of BTK in CLL. Furthermore, pharmacological or small interfering RNA–mediated targeting of HDAC1 activity increased the expression of BTK-targeting miRNAs and consequently reduced BTK expression and downstream signaling (see figure). Importantly, these reductions were evident in BTK(C481S) mutated samples, indicating this approach may hold potential for the treatment of patients who become resistant to ibrutinib. Indeed, the authors demonstrated the therapeutic potential of combining ibrutinib and HDACi’s in vitro on primary CLL cells and in vivo using the Eµ-TCL-1 mouse model, whereby responses were superior to either agent alone. Importantly, computational biology also predicts that preexisting drug-resistant clones may exist in patients prior to ibrutinib treatment.8 This indicates that the combination of HDACi’s with ibrutinib at least at therapy initiation may prevent/inhibit expansion of resistant clones during ibrutinib therapy, perhaps resulting in longer-term progression-free survival (PFS). However, there are some potential concerns associated with targeting HDACs in CLL. First, HDACi’s have known toxicity in CLL patients9 which may limit the usefulness of this approach. In particular, 1 side effect from treatment with HDACi’s is thrombocytopenia; therefore, how this drug combination will affect bleeding-related events, particularly if long-term usage in combination with ibrutinib is necessary, consequently requires further investigation. As a result, can HDACi’s be used at reduced doses to reduce toxicity to patients, while retaining their biological effect on miRNA expression? Can we use HDACi’s for only a short period of time and only if resistance to ibrutinib emerges? Or is HDACi treatment required prior to ibrutinib treatment? Indeed, Mato et al suggested an alternative KI may prove effective after resistance has emerged, although in that study overall response rate to a subsequent KI of 50% and a median PFS of 11.9 months suggested alternative salvage therapies still require investigation.10 In addition to the BTK(C481S) mutation, progressive disease in CLL while on ibrutinib can alternatively be attributed to PLCγ2 mutations (R665W and L845F) which promote BCR-downstream signaling independently of BTK. But how HDACi treatment will affect signaling/resistance associated with PLCγ2 mutations and whether this will select for PLCγ2 mutations in patients while on treatment still remains to be evaluated.
In conclusion, despite these unresolved queries, this pioneering study identifies that epigenetic silencing of BTK-targeting miRNAs may contribute to BTK overexpression in CLL and demonstrates the possibility of using HDACi’s to remove/reduce the emergence of ibrutinib-resistant clones. This study highlights the need to initiate clinical trials to assess this combination, given the urgent and unmet clinical need of kinase-resistant CLL patients at present.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal