Key Points
MRD negativity is a predictor for long-term progression-free and overall survival independent of the type and line of therapy.
MRD negativity confers the greatest prognostic benefit when achieved in the frontline setting.
Abstract
Minimal residual disease (MRD) negativity, defined as <1 chronic lymphocytic leukemia (CLL) cell detectable per 10 000 leukocytes, has been shown to independently predict for clinical outcome in patients receiving combination chemoimmunotherapy in the frontline setting. However, the long-term prognostic value of MRD status in other therapeutic settings remains unclear. Here, we retrospectively analyzed, with up to 18 years follow-up, all patients at our institution who achieved at least a partial response (PR) with various therapies between 1996 and 2007, and received a bone marrow MRD assessment at the end of treatment according to the international harmonized approach. MRD negativity correlated with both progression-free survival (PFS) and overall survival (OS) independent of the type and line of treatment, as well as known prognostic factors including adverse cytogenetics. The greatest impact of achieving MRD negativity was seen in patients receiving frontline treatment, with 10-year PFS of 65% vs 10% and 10-year OS of 70% vs 30% for MRD-negative vs MRD-positive patients, respectively. Our results demonstrate the long-term benefit of achieving MRD negativity, regardless of the therapeutic setting and treatment modality, and support its use as a prognostic marker for long-term PFS and as a potential therapeutic goal in CLL.
Introduction
Residual chronic lymphocytic leukemia (CLL) often remains in patients who have achieved a complete remission (CR) as defined in the International Workshop on CLL (IWCLL) response criteria.1 The expansion of residual CLL cells may lead to eventual disease relapse with the duration of remission dependent on the depth of remission and the rate of CLL repopulation. Minimal residual disease (MRD) can now be reliably detected to a level of 1 CLL cell in 104 leukocytes (0.01%). Although not routinely performed in clinical practice, many trials have assessed MRD levels using the flow cytometry assay harmonized and validated by the European Research Initiative on CLL.2-4
These trials have shown consistent correlation between posttreatment MRD level and therapeutic outcome,5-15 with MRD status demonstrating independent prognostic significance in patients treated upfront with chemoimmunotherapy.9,13,14 However, the independent prognostic relevance and long-term benefit of MRD negativity in other therapeutic settings, such as with chemotherapy-free treatments, remain unclear. Moreover, a direct comparison of the clinical impact of MRD negativity between frontline and relapsed/refractory settings has not hitherto been undertaken.
For the past 20 years, MRD evaluation has formed an integral part of the response assessment for CLL studies at our institution. This resulted in the availability of patients at different disease stages with known MRD status after various treatments, many with extended follow-up. We herein present an analysis of this historical cohort to address the long-term prognostic value of MRD status across different therapeutic settings and treatment modalities.
Patients and methods
Study criteria
We retrospectively analyzed all patients at our institution who completed treatment of CLL during 1996 to 2007, achieved at least a PR, and received a bone marrow MRD assessment within 6 months of treatment completion. Patients who failed to respond or died before treatment completion were excluded, as were those who received allogeneic stem cell transplantation, because graft-versus-leukemia effect can lead to continued depletion of residual disease. Also excluded were patients who subsequently received alemtuzumab for consolidation as part of the National Cancer Research Institute (NCRI) CLL207 trial. For individuals receiving multiple treatments, the first therapy completed between 1996 and 2007 was used for analysis. This study was undertaken with approval from our institutional ethics committee, and informed consent was obtained from all patients.
MRD and outcome analysis
MRD assessments were carried out using multiparameter flow cytometry according to the international harmonized approach.2-4 Assessments performed before 2003 did not necessarily contain all the reported markers, but data were included only if it was ascertained from the pretreatment material that a limit of detection of ≤10−4 CLL cells could be achieved with the available markers. Progression evaluations were made blinded to MRD status, and MRD status did not influence treatment duration, except for individuals receiving alemtuzumab who were treated until maximum IWCLL and MRD response was attained.
Survival analysis was carried out using SPSS Statistics. OS and PFS were calculated from the date of treatment completion to death or clinical progression, respectively. Statistical significance in time-to-event analyses was evaluated using the univariate log-rank method. Multivariate analyses were performed using the Cox proportional-hazards model, and all prognostic variables that were routinely assessed and available for ≥70% of patients were included.
Results and discussion
Altogether, 536 patients were assessed at our MRD laboratory during the study period, of whom 173 received treatment at our institution. Of these, 23 were excluded because of a lack of CR/PR, 10 owing to the assessment of blood rather than bone marrow MRD, 3 because of treatment with allogeneic stem cell transplantation, and 4 as a result of subsequent enrollment into a consolidation trial (NCRI CLL207). Among the 133 patients who fulfilled the inclusion criteria, 67 received combination chemotherapy or chemoimmunotherapy, 31 received single-agent chemotherapy, 7 underwent autologous stem cell transplantation, and 28 were treated with chemotherapy-free regimens, mostly with monoclonal antibody therapy. Fifty-seven received no previous CLL treatment, with the remainder having 1 to 7 prior therapies. Fifty-five (41%) were MRD-negative post treatment, including 46 with CR/CRi and 9 with PR/nPR. IWCLL response was evaluated in most cases (78%) by CT imaging. All patients with a MRD-negative PR had morphologically clear bone marrow but residual adenopathy. The demographic and treatment details are summarized in supplemental Tables 1 and 2, available on the Blood Web site.
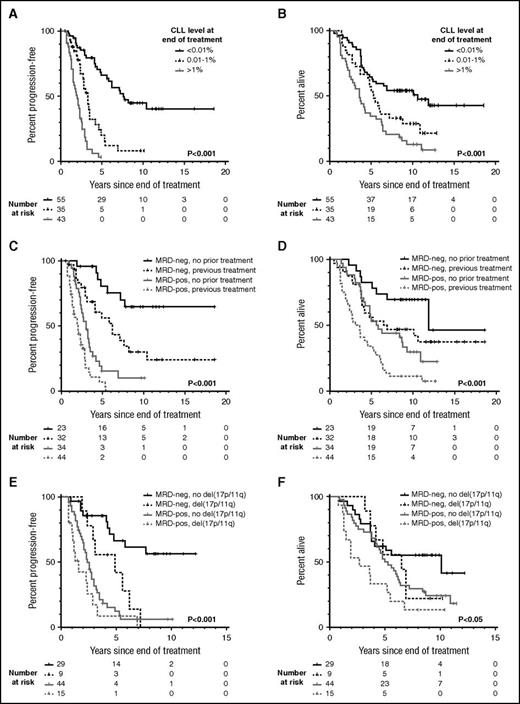
With a median follow-up of 10.1 years (range, 7.8-18.6) among surviving patients, the median PFS in MRD-negative (< .01%) individuals was 7.6 years, compared with 3.3 and 2 years, respectively, in individuals with positive MRD at 0.01% to 1% and >1% (Figure 1A). The median OS was likewise prolonged in MRD-negative patients (10.6 years) compared with MRD-positive patients (5.3 and 3.6 years, respectively for 0.01% to 1% and >1% MRD; Figure 1B). Patients with MRD-negative PR appeared to have outcomes intermediate between patients with MRD-negative CR and those with MRD-positive CR/PR (supplemental Figure 1). When MRD response was considered together with established prognostic factors including age, Binet stage, cytopenias, prior treatment, and adverse cytogenetics evaluated at the time of treatment, as well as treatment modality and IWCLL response, only MRD response and adverse cytogenetics were significant for PFS, and only MRD response, age, stage, and prior treatment were significant for OS on multivariate analysis (Table 1).
Presence of residual disease at the end of treatment predicts for long-term PFS and OS independent of prior treatment and cytogenetics. Posttreatment minimal residual disease (MRD) levels were obtained within 6 months after the end of treatment by multiparameter flow cytometry to a sensitivity of 10−4 (0.01%). A patient was considered MRD-negative if the MRD level was below the level of detection (ie, <0.01%). The log-rank P value is displayed, and P < .05 is considered statistically significant. (A) Progression-free survival (PFS) according to the level of detectable disease at the end of treatment. (B) Overall survival (OS) according to the level of detectable disease at the end of treatment. (C) PFS according to prior treatment and the MRD status at the end of treatment. (D) OS according to prior treatment and the MRD status at the end of treatment. (E) PFS according to del(17p) or del(11q) and the MRD status at the end of treatment. (F) OS according to del(17p) or del(11q) and the MRD status at the end of treatment. (E-F) Cytogenetic aberrations were evaluated by fluorescence in situ hybridization. The balance of patients with del(17p) and del(11q), respectively, was comparable between the MRD-negative and MRD-positive groups. In the MRD-negative del(17p/11q) group, 3 of 9 patients (33%) had del(17p), whereas 6 of 9 patients (67%) had del(11q). In the MRD-positive del(17p/11q) group, 6 of 15 patients (40%) had del(17p), whereas 9 of 15 patients (60%) had del(11q). MRD-neg, MRD-negative; MRD-pos, MRD-positive.
Presence of residual disease at the end of treatment predicts for long-term PFS and OS independent of prior treatment and cytogenetics. Posttreatment minimal residual disease (MRD) levels were obtained within 6 months after the end of treatment by multiparameter flow cytometry to a sensitivity of 10−4 (0.01%). A patient was considered MRD-negative if the MRD level was below the level of detection (ie, <0.01%). The log-rank P value is displayed, and P < .05 is considered statistically significant. (A) Progression-free survival (PFS) according to the level of detectable disease at the end of treatment. (B) Overall survival (OS) according to the level of detectable disease at the end of treatment. (C) PFS according to prior treatment and the MRD status at the end of treatment. (D) OS according to prior treatment and the MRD status at the end of treatment. (E) PFS according to del(17p) or del(11q) and the MRD status at the end of treatment. (F) OS according to del(17p) or del(11q) and the MRD status at the end of treatment. (E-F) Cytogenetic aberrations were evaluated by fluorescence in situ hybridization. The balance of patients with del(17p) and del(11q), respectively, was comparable between the MRD-negative and MRD-positive groups. In the MRD-negative del(17p/11q) group, 3 of 9 patients (33%) had del(17p), whereas 6 of 9 patients (67%) had del(11q). In the MRD-positive del(17p/11q) group, 6 of 15 patients (40%) had del(17p), whereas 9 of 15 patients (60%) had del(11q). MRD-neg, MRD-negative; MRD-pos, MRD-positive.
Univariate and multivariate analysis of posttreatment MRD levels with other parameters of prognostic significance
| Parameter . | Progression-free survival . | Overall survival . | ||||
|---|---|---|---|---|---|---|
| Univariate (log-rank) P . | Multivariate (Cox) P . | Hazard ratio (95% CI) . | Univariate (log-rank) P . | Multivariate (Cox) P . | Hazard ratio (95% CI) . | |
| Age* (60 y) | .513 | .001 | .001 | 2.41 (1.45-4.00) | ||
| Hemoglobin* (110 g/L) | .957 | .058 | ||||
| Platelet* (100 × 109/L) | .001 | .983 | .034 | .168 | ||
| Binet stage* (A/B vs C) | .005 | .870 | .001 | .018 | 2.23 (1.14-4.33) | |
| Prior treatment (Y/N) | .003 | .159 | .003 | <.001 | 2.61 (1.61-4.23) | |
| Treatment type | <.001 | .265 | .004 | .886 | ||
| IWCLL response | <.001 | .545 | .001 | .585 | ||
| MRD level (<0.01/0.01-0.1/0.1-1/>1%) | <.001 | <.001 | 2.07 (1.59-2.69) | <.001 | .002 | 1.39 (1.13-1.70) |
| Adverse cytogenetics* (del 17p/11q)† | .024 | .013 | 2.00 (1.16-3.45) | .051 | ||
| Parameter . | Progression-free survival . | Overall survival . | ||||
|---|---|---|---|---|---|---|
| Univariate (log-rank) P . | Multivariate (Cox) P . | Hazard ratio (95% CI) . | Univariate (log-rank) P . | Multivariate (Cox) P . | Hazard ratio (95% CI) . | |
| Age* (60 y) | .513 | .001 | .001 | 2.41 (1.45-4.00) | ||
| Hemoglobin* (110 g/L) | .957 | .058 | ||||
| Platelet* (100 × 109/L) | .001 | .983 | .034 | .168 | ||
| Binet stage* (A/B vs C) | .005 | .870 | .001 | .018 | 2.23 (1.14-4.33) | |
| Prior treatment (Y/N) | .003 | .159 | .003 | <.001 | 2.61 (1.61-4.23) | |
| Treatment type | <.001 | .265 | .004 | .886 | ||
| IWCLL response | <.001 | .545 | .001 | .585 | ||
| MRD level (<0.01/0.01-0.1/0.1-1/>1%) | <.001 | <.001 | 2.07 (1.59-2.69) | <.001 | .002 | 1.39 (1.13-1.70) |
| Adverse cytogenetics* (del 17p/11q)† | .024 | .013 | 2.00 (1.16-3.45) | .051 | ||
Bold values are statistically significant (P < .05).
Age, hemoglobin, and platelet count; Binet stage; and cytogenetics were assessed at the time of treatment initiation.
Cytogenetic aberrations [del(17p) and/or del(11q)] were evaluated by metaphase fluorescence in situ hybridization.
Patients receiving both frontline and subsequent treatments derived significant PFS and OS benefit from attaining MRD negativity (Figure 1C-D). However, greater long-term benefit was seen when MRD negativity was achieved upfront, with 10-year PFS of 65% vs 10% and 10-year OS of 70% vs 30% for MRD-negative vs MRD-positive patients. In comparison, in the relapsed/refractory setting, the 10-year PFS was 30% vs 0% and 10-year OS was 47% vs 11% for MRD-negative vs MRD-positive patients. The PFS curve for the 23 patients who achieved MRD negativity upfront appears to plateau at 7.7 years, beyond which no clinical relapse was observed among the 12 (52%) who remained in remission (Figure 1C). This is similar to the PFS plateau reported in the IGHV-mutated MRD-negative patients from the MD Anderson Cancer Center fludarabine, cyclophosphamide, and rituximab (FCR) trial,16 although in our cohort, 1 of 9 patients with known IGHV status in remission beyond 7.7 years had unmutated IGHV. Finally, in patients with del(17p) or del(11q), achievement of MRD negativity appeared to partially overcome the poor prognosis associated with their cytogenetic aberrations (Figure 1E-F), suggesting that targeting MRD may potentially be of value in this patient group.
This is the first study to incorporate different treatment settings and modalities into a single multivariate model to evaluate the clinical impact of posttreatment MRD status in CLL. In line with IWCLL guidelines,1 bone marrow rather than peripheral blood MRD was reported, because bone marrow is considered a more sensitive site for MRD detection than blood early after treatment completion, particularly in patients receiving monoclonal antibodies.2 Our study confirms the independent predictive value of MRD not only in the frontline but also in the relapsed/refractory setting, and not only with chemoimmunotherapy but also with chemotherapy-free treatments. Extended follow-up of our cohort revealed long-term treatment-free remissions among MRD-negative patients, particularly when MRD negativity was attained upfront. Hence, our findings support MRD as a prognostic marker for long-term PFS. Indeed, the use of MRD as a trial end point is currently under active investigation. An explanation for the importance of achieving MRD negativity upfront could be that poor survival is associated with the development of a resistant genotype (eg, TP53 mutation), which arises or is enriched within residual disease post therapy.17-19 In patients with profound remissions, there is a smaller pool of residual cells in which such resistance can occur. This underscores the importance of achieving the best possible response with first-line therapy.
Because of the historical nature of our cohort, IGHV mutational status was not available in every patient. Moreover, some therapies in our cohort were historical. However, the significance of this study lies in the demonstration that the clinical benefit of MRD negativity was independent of the type or line of therapy through which this was achieved. Indeed, the median PFS of the MRD-negative and MRD-positive patients in our cohort treated with chemotherapy-free regimens was significantly different at 4.9 and 1.3 years, respectively (P = .002). Currently, MRD negativity is achieved predominantly through chemotherapy-containing regimens with considerable toxicity, thus precluding its use in frailer patients with CLL. Newer agents such as venetoclax can also produce MRD negativity in substantial proportions of CLL patients, including in individuals with del(17p).20,21 The prognostic significance of MRD with novel treatments will need to be prospectively validated. In the future, chemotherapy-free combinations may potentially allow MRD eradication with minimal toxicity, making MRD negativity a feasible therapeutic goal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the staff of the Haematological Malignancy Diagnostic Service in Leeds, where the laboratory analysis was performed; the numerous clinicians and nurses at our institution and associated hospitals who treated the patients; and the patients who participated in this study.
Authorship
Contribution: M.K. and A.C.R. designed the study, collected and analyzed data, and wrote the manuscript; A.V., P.A.S.E., S.J.M.O., C.D., D.J.N., and P.M. provided advice and assisted with data collection; and P.H. conceived the study and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Hillmen, Department of Haematology, St. James’s Institute of Oncology, Bexley Wing, St. James’s University Hospital, Beckett St, Leeds, United Kingdom; e-mail: peter.hillmen@nhs.net.
References
Author notes
M.K. and A.C.R. contributed equally to this study.