Abstract
Hematopoietic stem cells (HSCs) have long been considered the continuous source of all hematopoietic cells for the life of an individual. Recent findings have questioned multiple aspects of this view, including the ability of lifelong HSCs to contribute to tissue-resident immune cells. Here we discuss the most recent findings on the source of B1a cells, innatelike lymphocytes that primarily reside in serous cavities. Powerful experimental approaches including bar coding, single cell transplantation, in vivo lineage tracing, and HSC-specific pulse-chase labeling have provided novel insights on B1a-cell generation during ontogeny. We evaluate the evidence for fetal vs adult B1a-cell production capacity and the identity of putative cells of origin. Integrating these most recent findings with previous work, we propose a working model that encapsulates our current understanding of waves of immune development.
Introduction
Challenges to the paradigm of HSCs as the source of all hematopoietic cells
Hematopoietic stem cells (HSCs) were first conceptualized by a series of experiments performed by Till, McCulloch, and colleagues more than 50 years ago based on their ability to give rise to clonal hematopoietic colonies upon transplantation.1-3 Since then, the isolation and characterization of purified HSCs from both adult bone marrow (BM) and fetal tissues has demonstrated their ability to sustain self-renewal and robust multilineage readout at the single-cell level upon transplantation.4 The advent of lineage-tracing models to study HSC function further supported the sustained contribution of labeled HSCs to both self-renewing HSCs and to mature peripheral blood lineages in situ.5,6 Together, amassed data utilizing these approaches contributed to the prevailing view that HSCs reside at the top of the hematopoietic hierarchy and are responsible for production of blood and immune cells across the life span of the individual. Several recent findings have questioned multiple aspects of this view, including the contribution of HSCs to the tremendous number of cells generated every day to maintain homeostasis,7,8 as well as the ability of lifelong HSCs to contribute to tissue-resident immune cells that are mainly specified early in life.9 Recent investigation into the ontogeny of tissue-resident macrophages has strongly suggested that the generation of at least some of these cell types is restricted to fetal development. These data have called into question whether particular immune cell subsets can be generated in adulthood, either under normal homeostatic conditions or stress, and whether HSCs are the source of the initial development or continuous replenishment of all types of immune cells.
Discovery of innatelike atypical B cells
The distinct lymphoid potential of fetal cells was first reported by the Herzenberg laboratory more than 3 decades ago.10 Adult BM cells were found to lack the capacity to regenerate immunoglobulin M–expressing “Ly-1” B cells upon transplantation, whereas this capability was retained by neonatal cells.10 This seminal finding has since been expanded to provide a better understanding of the unique functions of Ly-1 B cells, now referred to as B1a cells, as intermediaries between innate and adaptive immune function.11 In contrast to conventional B2 cells, the B-cell receptors of B1a cells have highly restricted immunoglobulin repertoires that often lack n-junction insertions, consistent with their development prior to postnatal expression of the enzyme terminal deoxynucleotidyl transferase.11,12 B1a cells express “natural” low-affinity antibodies with broad-specificity antigens including self-antigens and molecules expressed by pathogens. As such, B1a cells play a critical role in the rapid response to neonatal infections and are implicated in self-tolerance and autoimmunity.11,13
Despite greater understanding of the immune function of B1a cells, the origin and ontogeny of these unique B cells remains ambiguous. Over the years, some reports have confirmed that adult BM cells or HSCs regenerate B1a cells with far lower efficiency as compared with fetal cells (Figure 1A).14,15 Others have reported comparable B1a repopulation capability of adult and fetal HSCs, although differential dependence on interleukin-7rα (IL-7rα) signaling was suggestive of distinct waves of B-cell development.16 Examination of yolk sac hematopoiesis suggested that the initial waves of B1-specific progenitors arise in the early embryo, prior to and independent of HSC establishment.17,18 Although B1-specific progenitors have been identified during both fetal and adult hematopoiesis,19,20 B1a potential in adults may be reduced at the level of progenitor commitment.21 These findings, as well as the rapidly growing understanding of the origins of tissue-resident macrophages, have sparked intensified investigation into B1a specification. In this review, we take a closer look at 5 recent publications that address the origin, ontogeny, and developmental regulation of B1a cell production.
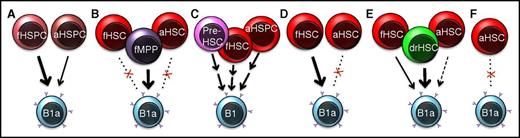
Proposed sources of tissue-resident B1a cells. The cellular origins of B1a cells have been debated since they were first identified as atypical B cells with unique functions and origins. (A) Early experiments led to the conclusion that fetal hematopoietic cells have superior B1a reconstitution capability compared with adult cells.10,14 (B) In 2016, Ghosn et al reported that the majority of B1a repopulation potential is provided by fetal putative progenitor cells as opposed to strictly defined fetal liver HSCs.22 (C) Montecino-Rodriguez et al, using a PU.1 hypomorph model, concluded that the first B1-cell generation is established prior to the existence of engraftable HSCs, with additional waves contributing during fetal and adult life.29 (D) Kristiansen et al used bar coding and single-cell transplantation31 to demonstrate that fetal HSCs are clonally capable of B1a-cell generation upon transplantation into adult recipients, whereas adult HSCs contributed little to B1a repopulation. (E) Beaudin et al showed that a small pool of drHSCs are uniquely apt to generate B1a cells compared with coexisting fetal liver HSCs, or adult BM HSCs.32 (F) Sawai et al used an HSC-specific pulse-labeling strategy to show that adult HSCs contribute minimally to B1a cells under homeostatic or interferon-challenged conditions in situ.35 HSPC, hematopoietic stem and progenitor cell; MPP, multipotent progenitor cell. An “f” prefix indicates fetal, an “a” indicates adult, and “dr” indicates developmentally restricted.
Proposed sources of tissue-resident B1a cells. The cellular origins of B1a cells have been debated since they were first identified as atypical B cells with unique functions and origins. (A) Early experiments led to the conclusion that fetal hematopoietic cells have superior B1a reconstitution capability compared with adult cells.10,14 (B) In 2016, Ghosn et al reported that the majority of B1a repopulation potential is provided by fetal putative progenitor cells as opposed to strictly defined fetal liver HSCs.22 (C) Montecino-Rodriguez et al, using a PU.1 hypomorph model, concluded that the first B1-cell generation is established prior to the existence of engraftable HSCs, with additional waves contributing during fetal and adult life.29 (D) Kristiansen et al used bar coding and single-cell transplantation31 to demonstrate that fetal HSCs are clonally capable of B1a-cell generation upon transplantation into adult recipients, whereas adult HSCs contributed little to B1a repopulation. (E) Beaudin et al showed that a small pool of drHSCs are uniquely apt to generate B1a cells compared with coexisting fetal liver HSCs, or adult BM HSCs.32 (F) Sawai et al used an HSC-specific pulse-labeling strategy to show that adult HSCs contribute minimally to B1a cells under homeostatic or interferon-challenged conditions in situ.35 HSPC, hematopoietic stem and progenitor cell; MPP, multipotent progenitor cell. An “f” prefix indicates fetal, an “a” indicates adult, and “dr” indicates developmentally restricted.
New advances
B1a production by atypical fetal HSCs
In a report22 published earlier this year, the Herzenberg Laboratory followed up on their previous studies by addressing which cells in the fetal liver are responsible for B1a generation. In light of recent reports that fetal B1a production may be HSC independent,17,18 Ghosn et al22 addressed which cells in the fetal liver are capable of B1a repopulation. Strictly defined fetal HSCs (ckithighLin−Sca1high [KLS] CD150+CD48−CD41−CD45+, with lineage markers consisting of Gr1, NK1.1, CD11b, CD3, B220, IL-7Rα, and Ter119) maintained robust reconstitution of red and white blood cell lineages over 6 months at levels comparable to unfractionated fetal liver cells. Despite high levels of reconstitution of peripheral CD19+ B cells, peritoneal B1b and B2 cells, and marginal zone splenic B cells, fetal HSCs failed to give rise to robust numbers of peritoneal cavity or splenic B1a cells. In contrast, transplantation of 6000 CD150− KLS cells, previously designated as multipotent progenitor cells,23 yielded B1a output that was comparable to 1.5 million unfractionated fetal liver cells. These results led the authors to conclude that B1a cells likely develop from a fetal lineage independent of HSCs (Figure 1B). It should be noted, however, that fetal HSCs were previously found to exist in the CD11b+ fraction.24 The inclusion of CD11b in the lineage fraction may explain why Ghosn et al only observed long-term reconstitution in one-third of recipients of 100 HSCs. Importantly, although residing outside the fraction most enriched for functional HSCs, as defined by SLAM expression,23 fetal CD150− KLS cells retained long-term multilineage reconstitution (LTMR) capability, as evidenced by sustained granulocyte/macrophage and B- and T-cell output 7 months posttransplantation. Thus, not all HSCs were contained within the fraction Ghosn et al designated as LT-HSCs. These data underscore the previously reported heterogeneity of the fetal HSC compartment25,26 and further imply that B1a potential may exist in a distinct lineage, as suggested by the Herzenbergs several decades ago.27
B1a production by pre-HSCs
The notion that B1a cells can develop independently of classical HSCs is also supported by a recent report from the Dorshkind laboratory. As previous evidence suggested that PU.1 differentially regulates B1 and B2 lymphopoiesis,28 the authors dissected changes in the B1 lineage across fetal development in a PU.1 hypomorph mouse model, generated by deletion of an upstream regulatory element (URE) of Sfpi1 (UREΔ/Δ mice).29 Using a combination of transplantation and in vitro colony assays, they observed differential output of B1 and B2 cells in UREΔ/Δ compared with wild-type mice at different stages of development. As the URE deletion affected both B1a and B1b development, the authors focused primarily on B1 progenitor production as a proxy for B1 cell readout. Whereas fetal B1 progenitor development was delayed in UREΔ/Δ mice, seemingly because of the absence of B1 production from pre-HSCs, adult B1 development was not affected. Building on evidence from previous reports,17,18 the authors concluded that B1 cells first develop from a stage prior to the existence of HSCs, and that stage-specific differences in B1 output reflect distinct waves of fetal progenitors with either B1 potential only or combined B1/B2 potential (Figure 1C). To resolve these waves at the molecular level, expression analysis was performed on fetal, neonatal, and adult progenitor cells. These analyses found that putative B1 and B2 progenitors cluster distinctly and further suggested that fetal and neonatal B2 progenitors may be distinct from adult B2 progenitors. These data provide additional support for successive waves of lymphopoiesis, distinguished in part by their differential reliance on PU.1 expression, and evidence that the earliest B1 lineage cells are specified prior to the existence of engraftable HSCs.
B1a production by clonal HSCs from fetal liver but not adult BM
Whereas adult HSCs have been shown to possess limited B1a output upon transplantation,14,15 Yuan et al previously demonstrated that expression of Lin28b was sufficient to restore fetal lymphopoiesis in adult HSCs, including endowment of robust B1a potential.30 In their most recent publication, the Yuan group employed cellular bar coding to test the ability of clonal HSCs to give rise to B1a cells.31 They demonstrated that B1a potential coincides with both B2 and granulocyte generation in the majority of bar-coded fetal liver, but not BM, KLS cells (Figure 1D). These same fetal cells exhibited both sustained granulocyte potential over 16 weeks posttransplantation and the greatest contribution to the B1a compartment, as determined by bar code read frequency. Sustained, robust trilineage reconstitution suggested that HSCs were responsible for most of the B1a output, although they also observed some contribution from progenitors (cells with B1a/B2 potential that lacked sustained granulocyte potential) to the B1a compartment. The concept that clonal HSCs give rise to B1a cells was also tested by transplantation of single fetal HSCs (defined as CD48−Flt3−CD150+ KLS cells). Although only 2 of 40 recipients were reconstituted at 7 weeks, robust reconstitution of B1a cells was evident in both cases, suggesting that B1a capability resides within the most engraftable HSC fraction. Secondary transplantation of sorted GFP+ KLS cells from a recipient of bar-coded fetal KLS cells revealed robust serial reconstitution capability of clones with combined granulocyte, B1a, and B2 potential, consistent with HSC function. Although B1a potential was maintained, overall B1a reconstitution was considerably lower in a secondary recipient compared with primary recipients. Kristiansen et al31 thus concluded that B1a potential is lost across ontogeny in HSCs that initially possessed that potential. To define the molecular mechanism regulating B1a potential across development, the authors revisited the correlation between B1a potential and Lin28b expression. Consistent with their previous work,30 forced expression of Lin28b in adult BM KLS cells resulted in both greater self-renewal capability and B1a potential. Collectively, the authors concluded that fetal HSCs possess B1a potential at the single-cell level and proposed that this potential is lost in adulthood because of intrinsic molecular regulation by the Lin28b/let7 axis.
B1a production by a distinct lineage of developmentally restricted HSCs
Our own recent results32 build on the framework that B1a capability is primarily restricted to fetal hematopoiesis and investigates the cellular mechanism underlying fetal origin. Using a lineage-tracing approach, we uncovered the existence of 2 coexisting fetal HSCs: 1 that persists into adulthood and 1 that does not. Both HSCs retain LTMR potential upon serial transplantation into conditioned adult hosts. In itself, the presence of 2 HSC populations is not surprising, given previous reports on both phenotypic24,26 and functional25 heterogeneity of the fetal HSC compartment. Strikingly, however, 1 of these 2 populations does not contribute to the adult HSC pool: the definitive nature of the FlkSwitch lineage-tracing model, an irreversible color switch from Tomato (Tom) to GFP driven by Flk2-mediated expression of Cre,33,34 established that the transient, developmentally restricted HSC (drHSC) present in the fetal liver and neonate BM does not contribute to the adult HSC compartment. Functional assays revealed that the drHSC has properties distinct from both the coexisting fetal HSCs and adult HSCs, as the GFP+ drHSC was lymphoid-biased with a specifically enhanced capacity to give rise to innatelike lymphocytes, including B1a cells. Quantitative comparison showed that although both fetal HSC populations had greater B1a potential than adult HSCs, the drHSCs had greater than threefold higher B1a output as compared with Tom+ fetal HSCs (Figure 1E). In addition to its distinct functional properties, the GFP+ drHSC was also phenotypically distinguished by expression of Flk2 and expressed lower levels of CD150 as compared with Tom+ HSCs. When these markers were used in combination with the Cre-mediated color switch, B1a potential was further enriched in the GFP+ KLS fraction. As the color-switching efficiency was incomplete during fetal development, the differences in B1a capability between Tom+ HSCs and GFP+ drHSCs were underestimated in these experiments. It is therefore possible that a distinct lineage of drHSCs contains all or most of the B1a potential. Thus, by defining a transient fetal HSC with B1a cell capability, this work advanced the concept of fetal HSC heterogeneity to include a transient, definitive lineage with unique differentiation potential.
Limited contribution to B1a cells by adult HSC in situ
The use of transplantation models to test B1a capability may not necessarily reflect stem/progenitor cell contribution in situ.7,8 To determine whether adult HSCs contribute to immune cells during unperturbed and interferon-induced inflammatory hematopoiesis, Sawai et al designed an HSC-selective inducible lineage-tracing strategy.35 Pulse labeling of adult HSCs with an inducible Map17-Cre mouse labeled a portion of adult cells characterized as HSCs by both label retention and serial reconstitution capability. Whereas contribution of labeled HSCs to most immune cells, including monocytes, dendritic cells, and B2 B cells, increased significantly over time, the labeling of tissue-resident immune compartments, including B1a cells, was low. Less than 5% labeling was observed in B1a cells 46 weeks post-HSC label induction, as compared with >50% for conventional circulating B cells. Labeling of B1a cells was also unchanged in response to challenge with polyinosinic:polycytidylic acid, despite an overall increase in contribution of labeled cells to B-cell progenitors and mature cells of other lineages. The authors argued that the far greater labeling of progenitor cells compared with that of B1a cells excludes adult HSCs as a source for the vast majority of B1a cells (Figure 1F), consistent with previous transplantation studies.14,15 These data demonstrated that adult HSCs and their progeny do not contribute significantly to B1a cell maintenance either in an unperturbed setting or upon interferon challenge and therefore provide convincing evidence that the B1a pool is established earlier in life.
Summary
Fetal HSCs have robust B1a repopulation capacity in adult recipients
The recent findings discussed in this review shed new light on the cellular origins of B1a cells and the mechanisms regulating their ontogeny. The limited ability of adult HSCs to produce B1a cells, both upon transplantation31,32 and during native hematopoiesis,35 is considerably strengthened by these recent reports. Thus, the long-standing notion that B1a cells, like tissue-resident macrophages and Vγ3-expressing dendritic epidermal T cells, are mainly specified during fetal life appears firmly cemented. However, the developmental timing and cellular source of tissue-resident lymphoid and myeloid cells appear to differ. Whereas at least some tissue macrophages appear to be of yolk sac origin and are poorly reconstituted by HSC transplantation,36-38 both Beaudin et al32 and Kristiansen et al31 provided compelling evidence that B1a production can be robustly generated by fetal liver HSCs (Figure 2). These experiments also established that, in contrast to fetal-restricted T cells32,39 and many tissue macrophages,36-38,40 B1a cells can be efficiently generated upon transplantation in adult recipients. As emphasized by Ghosn et al,22 this may be an important consideration for human immune reconstitution.
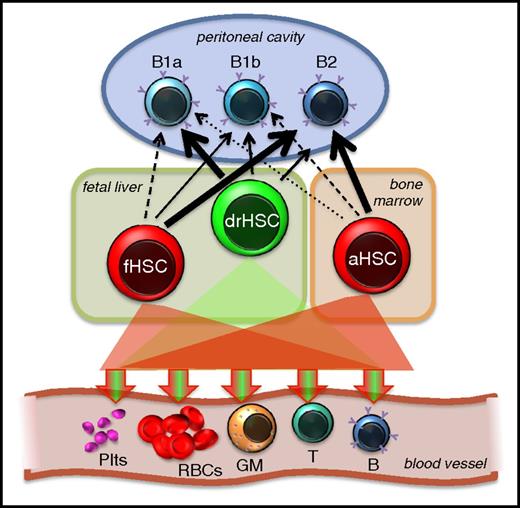
HSC contribution to B1a cells. All definitive HSCs, by definition, are capable of long-term multilineage contribution to the 5 main lineages of mature cells in adult recipients. Accumulating evidence converges on the concept that adult HSCs are poorer producers of B1a cells compared with fetal HSCs. Fetal HSC heterogeneity, which can be separated retrospectively by functional lineage bias classification,25 prospectively by surface markers24,26,32 or by lineage tracing,32 supports the notion that some fetal HSCs are more B1a competent than others and that transiently existing fetal HSCs account for most of the B1a capability. The HSC contribution to B1a cells emphasized here does not exclude the possibility that cells without robust engraftment capability (“pre-HSCs”) also contribute to the B1a pool. GM, granulocyte/macrophage; Plts, platelets; RBCs, red blood cells.
HSC contribution to B1a cells. All definitive HSCs, by definition, are capable of long-term multilineage contribution to the 5 main lineages of mature cells in adult recipients. Accumulating evidence converges on the concept that adult HSCs are poorer producers of B1a cells compared with fetal HSCs. Fetal HSC heterogeneity, which can be separated retrospectively by functional lineage bias classification,25 prospectively by surface markers24,26,32 or by lineage tracing,32 supports the notion that some fetal HSCs are more B1a competent than others and that transiently existing fetal HSCs account for most of the B1a capability. The HSC contribution to B1a cells emphasized here does not exclude the possibility that cells without robust engraftment capability (“pre-HSCs”) also contribute to the B1a pool. GM, granulocyte/macrophage; Plts, platelets; RBCs, red blood cells.
Dynamic heterogeneity of the fetal HSC compartment
Although there is considerable consensus for a predominantly fetal origin of B1a cells, there is not complete agreement on the cellular source. The Herzenberg and Dorshkind groups22,29 both concluded that B1a capability first arises in progenitor populations that lack properties expected of bona fide HSCs. Some of the differences in B1a output between different research groups may arise from differences in surface markers used to isolate the transplanted cell populations. Thus, although Ghosn et al22 concluded that certain fetal HSCs lack B1a capability, their observation of B1a output coinciding with LTMR outside of a conventional phenotypic HSC compartment is consistent with the phenotypic and functional heterogeneity of the fetal HSC compartment described in Beaudin et al.32 The finding from Ghosn et al that some HSCs lack B1a capacity supports the coexistence of 2 distinct lineages of fetal HSCs, 1 with strong B1a capacity and 1 with very little (Figure 2), that was reported in Beaudin et al. The notion of heterogeneity within the fetal HSC compartment fits well with parallel reports in adult hematopoiesis41,42 and also provides new support for the hypothesis initially proposed by the Herzenberg laboratory of sequentially developing HSCs that give rise to unique components of the immune system.43
Successive waves that shape the developing immune system
Our conclusion that fetal HSCs are major contributors to B1a cells does not exclude the existence of other sources, such as pre-HSCs, or the notion that several waves of lymphopoiesis contribute to B1a generation during fetal and neonatal development.29 The Reizis group35 showed that B1a contribution by adult HSCs is very low in situ but did not address contribution by fetal HSCs vs non-HSCs. It remains to be determined whether a “pre-HSC” wave of B1a production arises from the same progenitors that give rise to definitive HSCs with strong B1a potential. Intriguingly, some of the genes invoked by Montecino-Rodriguez et al29 as regulators of distinct waves, such as Flk2 and IL-7Rα, were also differentially regulated between lifelong and developmentally restricted HSCs.32,44 Thus, the 2 coexisting HSCs described in Beaudin et al32 may represent 2 distinct waves that succeed and partially overlap with the ones described by Dorshkind’s group.29 Together, these data bring greater clarity to understanding cellular drivers contributing to waves of lymphopoiesis. As we gain increasing insight into both waves of B-lymphopoiesis and greater appreciation for their functional heterogeneity, the question arises as to whether distinct waves generate distinct functional subsets. For example, is the small contribution to phenotypic B1a cells that is observed from adult HSCs, both upon transplantation32 and in situ,35 of physiological relevance, and are adult-derived B1a cells functionally equivalent to B1a cells specified earlier in life? Functional characterization of cells derived from different sources, such as pre-HSCs, fetal HSCs, and adult HSCs, will shed more light on the heterogeneity and physiological importance of B1a cells themselves, as well as the relationship and molecular regulation of their cellular origins.
Acknowledgments
The authors thank Susan Carpenter for insightful comments on the manuscript and Stephanie Smith-Berdan for assistance with illustrations.
Work related to this article was supported by a grant from National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100917) (E.C.F.); an Alex’s Lemonade Stand Foundation Innovation Award (E.C.F.); an American Asthma Foundation Research Scholar award (E.C.F.); a California Institute for Regenerative Medicine Training grant (TG2-01157) (A.E.B.); a National Institutes of Health, National Heart, Lung, and Blood Institute Mentored Career Development Award (K01HL130753) (A.E.B.); and California Institute for Regenerative Medicine Facilities awards (CL1-00506 and FA1-00617-1) to University of California, Santa Cruz.
Authorship
Contribution: A.E.B. and E.C.F. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: E. Camilla Forsberg, Institute for the Biology of Stem Cells, Department of Biomolecular Engineering, University of California, Santa Cruz, 1156 High St, SOE2, Santa Cruz, CA 95064; e-mail: cforsber@ucsc.edu.