Abstract
Background There is increasing evidence for an inherited predisposition to pediatric acute lymphoblastic leukemia (ALL). We and others have previously reported rare and highly penetrant variants in hematopoietic transcription factors (PAX5 and ETV6) and tumor suppressor genes (TP53) in both sporadic and familial ALL. IKZF1encodes the founding member of the Ikaros family of zinc finger transcription factors, and is a critical regulator of lymphoid development. IKZF1 is frequently targeted by somatic deletions and mutations in high-risk B-ALL, particularly Ph+ and Ph-like ALL, and is associated with poor outcome. IKZF1 alterations have previously been shown to result in the acquisition of stem cell-like features, overexpression of adhesion molecules causing aberrant cell-cell and cell-stroma interaction, and decreased sensitivity to tyrosine kinase inhibitors. Genome-wide association studies have also identified an association between common polymorphisms at the IKZF1locus and risk of developing ALL, however the nature and effects of germline IKZF1variation in the pathogenesis of ALL are poorly understood. In this study, we sought to comprehensively characterize germline IKZF1 genetic variation and to determine the extent to which they contribute to predisposition to ALL.
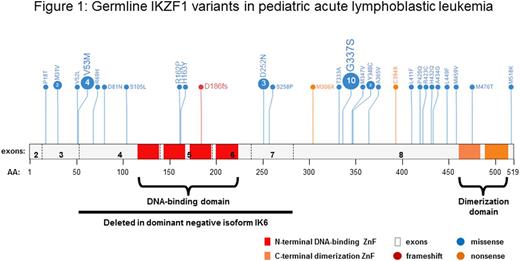
Methods We recently identified a germline frameshift IKZF1 variant (D186fs) in the proband of a family with BCR-ABL1 ALL with incompletely penetrant autosomal dominant inheritance, and carriers of this variant showed varying degree of B cell deficiency. We sequenced IKZF1in germline DNA from 5,008 children with ALL (4902 B-ALL and 106 T-ALL) enrolled on ChildrenÕs Oncology Group and St. Jude ChildrenÕs Res. Hosp. frontline ALL trials. We examined each variant for its effects on transcriptional repression, DNA-binding, cellular localization, homodimerization, and leukemic cell adhesion in mouse BCR-ABL1Arfnull B-ALL cells and/or in HEK 293T cells. All variants were assayed for their effects on cell viability and proliferation, cell-cell adhesion, and IKZF1 protein expression and localization in BCR-ABL1 Arfnull pre-B cells. Representative variants, including M31V (N-term), H163Y (DNA-binding domain), D186Tfs (familial index), M306* (truncation of C-terminus), and A434G (C-terminus) were also assayed in detail for their ability to dimerize with wild type IKZF1, bind to DNA, or dominant negative effects on transcription repressor activity in HEK293T cells. IKZF1 variants were also evaluated for inducing perturbations in cell adhesion and THY1, ITGA5, SELL expression in the mouse PreB cells, and adhesion within the bone marrow niche by ex vivo imaging of calvaria. Finally, the effects of variants on dasatinib sensitivity were assessedin vitro and in vivo.
Results We identified 28 germline IKZF1variants in children with ALL, mostly in B-ALL (Figure 1). Among these variants, 3 were frameshift or nonsense resulting in truncated IKZF1 proteins. Of the remaining missense variants, 2 were located within the N-terminal DNA-binding domain, 1 in the C-terminal dimerization domain, and 22 in other parts of IKZF1 protein with clustering proximal to the C-terminal zinc fingers. In mouse BCR-ABL1 Arfnull pre-B cells, all but 4 variants (P18T, P420Q, H432Q, and M518K) variably perturbed IKZF1 function. In contrast to expression of wild-type IKZF1, which caused growth arrest, 24 of the ALL variants were tolerated; 18 caused cellular aggregation; 15 displayed cytoplasmic mislocalization; and 14 out of 20 variants analyzed had significant upregulation of the adhesion molecules THY1, ITGA5 and/or SELL that are normally repressed by IKZF1. In HEK293T cells, 3 IKZF1 truncating variants showed dramatic loss of transcription repressor activity and no longer dimerized with wildtype IKZF1. DNA-binding domain variants (R162P, H163Y) failed to repress target promoter transcription but also altered wildtype IKZF1 function in a dominant negative fashion. In comprehensively characterization of representative variants (M31V, H163Y, D186Tfs, M306X, and A434G), these variations caused cell-stroma adherence in the bone marrow niche in vivo, and significantly reduced sensitivity of leukemic cells to dasatinib in vitro and in vivo.
Conclusions These results identify IKZF1 as a new ALL predisposition gene, and suggest that these germline risk variants have roles in both leukemia pathogenesis and treatment responsiveness.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal