Abstract
Background: The presence of circulating plasma cells (CPCs) is associated with an adverse prognosis in multiple myeloma along the entire spectrum of disease. However, the impact of CPCs prior to autologous stem cell transplantation (ASCT) has not been defined in the era of novel agents. The objective of this study is to assess the impact of the presence and number of CPCs before transplant using 6-color multiparameter flow cytometry (MFC). We also explored the additional prognostic implications of CPCs over the current paradigm for risk stratification at transplant, including high-risk (HR) cytogenetics and disease status at transplant by the IMWG criteria.
Methods: A total of 1113 consecutive patients transplanted between 2007 and 2015 in Mayo Clinic were included in the study. Flow-cytometry of peripheral blood to identify clonal CPCs is routinely performed in all patients before ASCT in Mayo Clinic, Rochester. The mononuclear cells from peripheral blood samples are isolated by Ficoll gradient and subsequently stained with antibodies to CD19, CD38, CD45, CD138 and cytoplasmic kappa (κ) and lambda (λ) immunoglobulin light chains. Clonality is assessed by light chain restriction (κ:λ expression ratio of 4:1 [κ restricted] or <1:2 [λ restricted]). The target for collection is >150,000 cellular events. For samples where less than 150,000 clonal events are examined, the number of final clonal events is adjusted to 150,000 events. HR cytogenetics by fluorescence in situ hybridization (FISH) on bone marrow (BM) plasma cells was defined by one of the following abnormalities: t(4;14), del(17p), t(14;16), t(14;20) or +1q, detected at any time point from diagnosis to transplant. Patients who had insufficient plasma cells for FISH analysis in BM specimens were not included in the denominator to calculate the percentage of patients with HR cytogenetics. The primary end-points of this study were post-transplant response, PFS and OS.
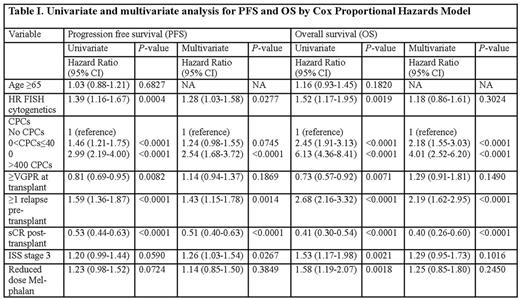
Result: CPCs were detected in 238 out of 1113 (21.4%) patients, with the median number of CPCs being 82 (range, 1-102381) per 150,000 events. The median age of patients at transplant was 62 years. A total of 1097 (99%) patients had received novel agent based induction therapy with PIs and IMiDs, including 458 (41%) patients who had received both PIs and IMiDs. 401 (36%) patients had received post-transplant maintenance or consolidation therapy. The frequency of t(4;14) in BMPCs was significantly higher in patients with CPCs compared to those without [15.2 vs 7.0% for t(4;14); P<0.001]. The median follow-up from transplant for survivors was 45 months. The 5-year OS rate in patients with and without CPCs was 34% (95% CI, 27-42) and 70% (95% CI, 66-74) respectively (P<0.001). After exploring several cut-offs for the number of CPCs per 150,000 events, we chose a simple pre-transplant risk stratification model: No CPCs (n=874; 78.5%), 0<CPCs≤400 (n=188; 16.9%) and CPCs>400 (n=51; 4.6%). The number of patients achieving stringent complete response post-transplant in groups with no CPCs, 0<CPCs≤400 and CPCs>400 was 310 (36%), 31 (16%) and 3 (6%) respectively (P<0.001). The median PFS in the 3 groups was 27 months (95% CI, 25-30), 15 months (95% CI, 13-18) and 6 months (95% CI, 4-10) respectively (P<0.001) and 5-year OS rates were 70% (95% CI, 66-74), 42% (95% CI, 34-51) and 12% (95% CI, 5-26) respectively (P<0.001). Kaplan-Meier curves for PFS and OS are depicted in Figure I (A and B). In a multivariate model (Table I), the groups with 0<CPCs≤400 and CPCs>400 had an increased risk of death, independent of the presence of HR cytogenetics and disease status at transplant.
Conclusion: Our study shows the strong negative prognostic impact of CPCs prior to transplant on post-transplant response and survival in MM in the era of novel agents. The proposed risk stratification model can be easily employed with simple equipment and should be routinely incorporated in the pre-transplant workup. Detailed epigenomic, genomic and transcriptomic analysis of CPCs is urgently needed to uncover the biological basis of resistance to both high-dose cytotoxic therapy and novel agents and identify potential avenues of targeted therapy against CPCs.
Kumar:Onyx: Consultancy, Research Funding; Kesios: Consultancy; Celgene: Consultancy, Research Funding; Glycomimetics: Consultancy; AbbVie: Research Funding; BMS: Consultancy; Sanofi: Consultancy, Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding. Dispenzieri:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; pfizer: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Jannsen: Research Funding. Kapoor:Celgene: Research Funding; Amgen: Research Funding; Takeda: Research Funding. Gertz:Sandoz Inc: Honoraria; NCI Frederick: Honoraria; Celgene: Honoraria; Alnylam Pharmaceuticals: Research Funding; GSK: Honoraria; Novartis: Research Funding; Research to Practice: Honoraria, Speakers Bureau; Med Learning Group: Honoraria, Speakers Bureau; Ionis: Research Funding; Prothena Therapeutics: Research Funding; Annexon Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal