Abstract
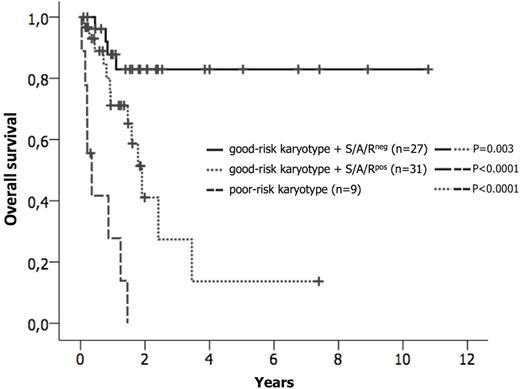
Systemic mastocytosis (SM) usually arises as a consequence of an acquired mutation in the receptor tyrosine kinase KIT, usually KIT D816V (>80-90% of patients). The presence of additional molecular aberrations, e.g. in SRSF2, ASXL, or RUNX1 (S/A/Rpos), has a strong adverse impact on disease phenotype and prognosis in patients with advanced SM (advSM) (Jawhar et al., Leukemia 30, 2016). However, little is known about the frequency and prognostic impact of cytogenetic aberrations and potential interactions between cytogenetic and molecular aberrations in the context of prognostication. We therefore analyzed clinical, morphological, cytogenetic and molecular characteristics of 109 patients (KIT D816V, n=102, 94%; KIT D816H, n=2, 2%; no KIT mutation, n=5, 5%) with indolent SM (ISM, n=27) and advSM (n=82) with or without an associated hematologic neoplasm (AHN) [SM-AHN, n=60; aggressive SM, n=3; mast cell leukemia (MCL), n=9; MCL-AHN, n=10)]. Next-Generation Deep Amplicon Sequencing (NGS) was performed to investigate 18 candidate genes as previously described (Schwaab et al., Blood 122, 2013). All ISM patients had a normal karyotype. In advSM, an aberrant karyotype was identified in 16/82 (18%) patients. By analogy with other myeloid neoplasms, e.g. MDS or AML, patients were classified according to their karyotype into two groups. The good-risk group (n=73) included patients with a normal karyotype (n=66) plus those with a favorable karyotype (n=7; del(5q), n=4; trisomy 8, n=1; del(1q), n=1; del(12p), n=1), while the poor-risk group (n=9) included patients with a complex karyotype (defined as ≥3 abnormalities; n=8) or monosomy 7 (n=1). Seven of 9 (78%) patients with a poor-risk karyotype and 2/7 (29%) patients with a favorable karyotype progressed to secondary AML (n=7) or MCL (n=2) within a median observation time of 13 months (range, 0-26). Overall, the median overall survival (OS) of patients with a poor-risk karyotype was significantly shorter than in patients with a good-risk karyotype (4 vs. 41 months; hazard ratio, HR, 9.6, 95% CI 3.9-23.2; P<0.0001). Of the 67 cases that were tested for gene mutations 5/9 (56%) with a poor-risk karyotype and 31/58 (53%) patients with a good-risk karyotype were S/A/Rpos. Significant differences regarding OS were observed when comparing the following 3 groups (Figure): good-risk karyotype + S/A/Rneg (n=27) vs. good-risk karyotype + S/A/Rpos (n=31) vs. poor-risk karyotype (n=9). In 6 multi-mutated advSM patients with simultaneous occurrence of cytogenetic [del(5q), n=3; trisomy 8, n=3], we explored the clonal architecture in single-cell-derivedgranulocyte-macrophage colonies (CFU-GM; median number per patient, n=12; range 10-15) using fluorescence in situ hybridization and mutation-specific PCR followed by Sanger sequencing. None of the colonies were positive for both KIT D816V and the cytogenetic aberration, indicating that these abnormalities marked different clones. However, some cytogenetically positive colonies were tested positive for additional mutations such as JAK2 V617F or TP53. In colonies without cytogenetic aberrations, additional mutations, e.g. SRSF2, ASXL1, RUNX1 or TET2, were identified with or without KIT D816V, confirming our previous results that the KIT D816V mutation is not the initial (earliest) event in SM evolution (Jawhar et al., Leukemia 29, 2015). We conclude, that a) molecular and cytogenetic analyses should be routinely performed in patients with advSM in addition to clinical and morphological baseline staging because they may more accurately indicate risk of disease progression/transformation and poor prognosis and b) patients with SM-AHN and an aberrant karyotype frequently harbor two different clones, with one responsible for SM evolution and the other driving AHN evolution independent of KIT D816V.
Kaplan-Meier estimates of overall survival (OS) in patients with advanced systemic mastocytosis depending on the karyotype and the mutation status in the SRSF2/ASXL1/RUNX1 gene panel (S/A/R). Pairwise significantly different OS probabilities were observed for the comparison good-risk karyotype + S/A/Rneg (n=27) vs. good-risk karyotype + S/A/Rpos (n=31) vs. poor-risk karyotype (n=9).
Kaplan-Meier estimates of overall survival (OS) in patients with advanced systemic mastocytosis depending on the karyotype and the mutation status in the SRSF2/ASXL1/RUNX1 gene panel (S/A/R). Pairwise significantly different OS probabilities were observed for the comparison good-risk karyotype + S/A/Rneg (n=27) vs. good-risk karyotype + S/A/Rpos (n=31) vs. poor-risk karyotype (n=9).
Valent:Novartis: Honoraria, Research Funding; Ariad: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria, Research Funding; Deciphera Pharmaceuticals: Research Funding. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Meggendorfer:MLL Munich Leukemia Laboratory: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal