Abstract
T cells recognizing solid tumors and Hodgkin's lymphoma can be released from anergy by immune checkpoint inhibitors. Its therapeutic success seems to be associated with the abundance of neoepitopes within the tumor mutanome. In neoplastic B cells, VDJ recombination and somatic hypermutation (SHM) generate unique immunoglobulin (Ig) peptide sequences that predictably contribute to the lymphoma mutanome. Efficient presentation of a neoepitope by an individual's HLA complex is an essential requirement for neoepitope-directed T-cell immunity. Presentation of Ig-derived peptides in HLA class I has hitherto been demonstrated only indirectly, and there is as yet no direct evidence for presentation of Ig-derived neoepitopes of primary human lymphoma cells.
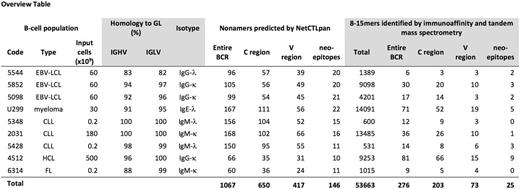
We analyzed HLA-presented Ig-derived peptides in 9 clonal B-cell populations: 3 chronic lymphocytic leukemias (CLL), 1 hairy cell leukemia, 1 follicular lymphoma (FL), 3 EBV-transformed lymphoblastoid cell lines, and the U299 myeloma cell line. Full-length B-cell receptor (BCR) VDJ and VJ sequences were obtained by unbiased ARTISAN PCR and Pacific Biosciences next generation sequencing. 1067 unique Ig-derived nonamers were predicted to bind the HLA class I alleles expressed by the respective B-cell clone by the NetCTLpan bioinformatics tool. 650 candidate epitopes (60.9%) were derived from constant (C) regions; 417 (39.1%) from variable (V) regions. 146 predicted peptides from V regions (35.0%) contained at least one amino acid change due to SHM or at least one amino acid from CDR3 and were therefore considered potential neoepitopes.
To investigate experimentally which epitopes are processed intracellularly and presented by HLA class I, HLA class I-peptide complexes were immunoaffinity purified by the monoclonal antibody W6.23 and analyzed by liquid chromatography and tandem mass spectrometry. In total, 53,663 unique peptides (8-15mers) were identified by Uniprot matching with a Mascot Ion Score of >20. HLA ligandome sizes of individual B-cell populations ranged from 600 to 14,091 unique peptides per case. Within any HLA ligandome, 6 to 81 peptides were annotated as BCR-derived epitopes if they matched the individual BCR sequences. Of the total of 276 eluted BCR peptides, 203 (73.5%) where derived from C regions and 73 (26.5%) from V regions. 25 eluted peptides (range 0-10 per case) were derived from CDR3 regions or contained SHM-induced amino acid changes, thus fulfilling the definition of neoepitopes. Neoepitopes were detected in all cases with more than 109 cells as input material. Up to date, 4 neoepitopes have been synthesized and their mass spectra confirmed. Confirmation of all remaining neoepitopes is ongoing. No BCR-derived neoepitopes could be detected in an IGHV-unmutated CLL and the FL. These two cases had the lowest number of input cells and small overall ligandome sizes of only 600 and 1015 unique UniProt-matched peptides, respectively.
We demonstrate for the first time that primary neoplastic B cells process and present BCR-derived neoepitopes in the context of HLA class I. Despite their origin from only two polypeptides, BCR epitopes and neoepitopes represent app. 0.5% and 0.05% of the total HLA class I ligandome, respectively. The challenging identification of BCR neoepitopes was possible by unbiased identification of BCR transcripts combined with next generation sequencing and targeted search for HLA class I-bound peptides. Matching fragmentation patterns of native and synthetic peptides suggest high specificity of this strategy. With respect to sensitivity, the ability to identify low frequency peptides appears to be strongly dependent on the amount of input cells.
Our data close a gap in the mechanism underlying the evidence that highly immunogenic formulations of an idiotype vaccine are able to induce MHC class I-restricted cytotoxic T-cell responses. While phase III trials of idiotype vaccination aiming to induce anti-Ig antibodies failed to demonstrate convincing prolongation of clinical remissions achieved by chemotherapy, our data lend support for exploring idiotype-specific T-cell immunity against B-cell lymphomas. With recent advances in peptide synthesis, adjuvant formulations, and the availability of check point inhibitors to surpass regulatory activity, active immunotherapy targeting the lymphoma idiotype may regain appeal as truly personalized immune therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal