Abstract
Introduction: Guadecitabine (SGI-110) is a novel next-generation hypomethylating agent (HMA) administered as a small volume subcutaneous (SC) injection which results in extended decitabine exposure. Phase 2 study has been conducted in r/r AML patients using two different doses and schedules of guadecitabine. We report here long term survival and clinical complete response rates in various prognostic subgroups of r/r AML patients
Methods: r/r AML patients who were either refractory to or relapsed after induction chemotherapy were enrolled in this Phase 2 study. In the first cohort, patients were randomized (1:1) to either 60 mg/m2/d or 90 mg/m2/d on Days 1-5 (5-day regimen). In the second cohort, patients were assigned to treatment with 60 mg/m2/d on Days 1-5 and Days 8-12 (10-day regimen) for up to 4 cycles, followed by 60 mg/m2/d Days 1-5 in subsequent cycles. Cycles were scheduled every 28 days for both regimens with dose reductions/delays allowed based on response and tolerability. Patients remained on treatment as long as they continued to benefit without unacceptable toxicity. The primary endpoint was the composite Complete Response (CRc) rate: CR + CR with incomplete platelet recovery and normal neutrophils count (CRp) + CR with incomplete neutrophil recovery (CRi) using modified International Working Group (IWG) criteria (Cheson et al, 2003). Secondary endpoints included overall survival (OS), and safety as measured by Adverse Events (AEs) using CTCAE v4.0, and early 30 and 60-day all-cause mortality. Individual dose and schedule results were previously reported (Roboz et al, European Society of Medical Oncology, 2014) where there was no difference between the 2 doses of 60 and 90 mg/m2/d and a trend of higher CRc and CR for the 10-day initial intensification regimen. We report here long tem survival and CRc data in various prognostic subgroups in the overall r/r AML patient population treated with guadecitabine.
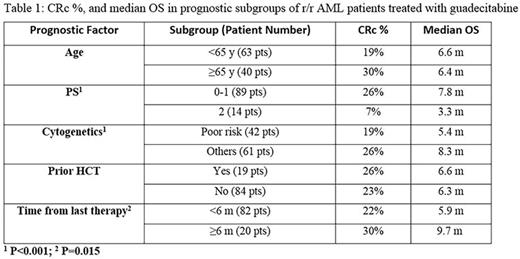
Results: 103 r/r AML patients were treated with guadecitabine (50 with 5-day regimen and 53 with the initial 10-day intensification regimen). Eight six patients (84%) received prior standard 7+3 induction; 15 patients (14%) received other prior induction chemotherapy regimens mostly with high dose cytarabine; clofarabine or cladribine with or without cytarabine; and only 2 patients (2%) received HMA as prior front line induction treatment. Median age was 60y (range 22-82y); male 60%; PS 2 in 14%; poor risk cytogenetics 41%; prior Hematopoietic Cell Transplant (HCT) 18%, and median number of 2 prior regimens (range 1-10) including 11% with prior HMA treatment. CRc was achieved in 24 patients (23%). After a median follow up of 29 months, the median OS was 6.6 months with 1-y and 2-y survival rates of 28 and 19% respectively. CRc was associated with statistically significant longer survival; median OS not reached for patients in CRc and was 5.6 months for patients with no CRc (p<0.01). Table 1 shows CRc rates and Median OS in some of the standard prognostic subgroups. Clinical activity in terms of CRc was observed in all subgroups with no statistically significant difference between any of the prognostic subgroups examined based on age; PS; cytogenetics risk; prior HCT; and time from last therapy. However OS was significantly worse in patients with PS 2 (p<0.001); patient with poor risk cytogenetics (p<0.001); and those with <6 months from last therapy (p=0.015). Safety was acceptable in all doses and schedules, the most common Grade ≥3 AEs regardless of relationship to therapy were febrile neutropenia (60%), pneumonia and thrombocytopenia (36% each), and anemia (31%). All-cause early mortality was relatively low for this patient population with 3.8% at 30 days and 11.6% at 60 days in all guadecitabine treated patients with no higher mortality with the 10-day initial intensification regimen.
Conclusions: Guadecitabine showed good clinically activity in terms of CRc with acceptable safety profile in r/r AML patients with various standard poor prognostic factors. CRc with guadecitabine was associated with longer survival. While CRc was observed in all subgroups, patients with poor PS; poor risk cytogenetics; or with short time from last therapy had significantly worse survival. A randomized phase 3 study is being initiated with guadecitabine using the 10-day initial intensification regimen vs Treatment Choice in r/r AML patients.
Daver:Sunesis: Consultancy, Research Funding; Ariad: Research Funding; Karyopharm: Honoraria, Research Funding; Pfizer: Consultancy, Research Funding; BMS: Research Funding; Kiromic: Research Funding; Otsuka: Consultancy, Honoraria. Kantarjian:Amgen: Research Funding; Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Roboz:Cellectis: Research Funding; Agios, Amgen, Amphivena, Astex, AstraZeneca, Boehringer Ingelheim, Celator, Celgene, Genoptix, Janssen, Juno, MEI Pharma, MedImmune, Novartis, Onconova, Pfizer, Roche/Genentech, Sunesis, Teva: Consultancy. Kropf:Celgene: Consultancy; Takeda: Consultancy. Yee:Novartis Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Berdeja:Abbvie, Acetylon, Amgen, Bluebird, BMS, Calithera, Celgene, Constellation, Curis, Epizyme, Janssen, Karyopharm, Kesios, Novartis, Onyx, Takeda, Tragara: Research Funding. Su:Astex Pharmaceuticals, Inc.: Employment. Azab:Astex Pharmaceuticals, Inc.: Employment. Issa:Astex Pharmaceuticals, Inc.: Consultancy; Teva: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal