Abstract
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by transient or persistent decrease of platelet count. ITP is the most common cause of thrombocytopenia in early pregnancy. Patients with severe thrombocytopenia (platelet count < 20 ×109/L) are at risk of spontaneous bleeding, postpartum hemorrhage and placental abruption. The aim of this study is to determine the efficacy and the safety of recombinant human thrombopoietin (rhTPO) in the management of ITP in pregnancy. This study is registered at www.clinicaltrials.gov as NCT02391272.
Patients at eight centers in China were enrolled. Enrollment criteria were pregnant women aged between 18 and 50, who failed to respond to first-line treatments of ITP and/or were refractory to platelet transfusion. Patients' platelet counts were below 30×109/L with bleeding manifestations. Gestational age of the patients was over 12 weeks. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
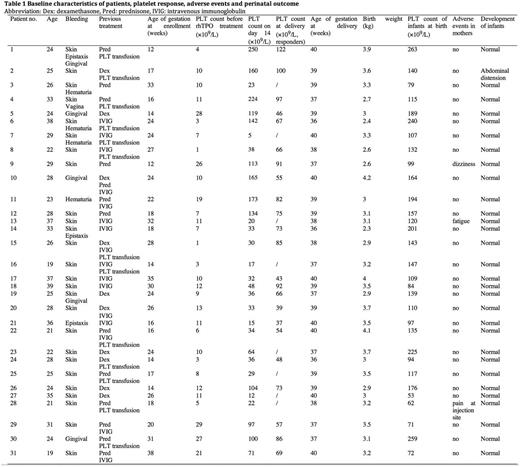
Thirty-one patients were enrolled into the study. The median age of the pregnant ITP patients was 26 years (range 19 - 39 years) and 90.6% (29/31) were primigravidae. The median gestational age was 24 weeks (12 - 38 weeks). The median baseline platelet count was 10×109/L (range 1 - 29×109/L). 74.2% (23/31) of these patients were diagnosed as ITP before pregnancy and 25.8 % (8/31) during pregnancy.
All eligible participants received rhTPO at an initial dose of 300U/kg once daily subcutaneously for 14 days, 74.2% (23/31) of these patients responded to the initial 14-day rhTPO therapy, including 10 CR and 13 R. Eight patients were NR though their platelet counts rose mildly. The median platelet count of responder was 100×109/L (range from 30 to 250×109/L) at day 14.
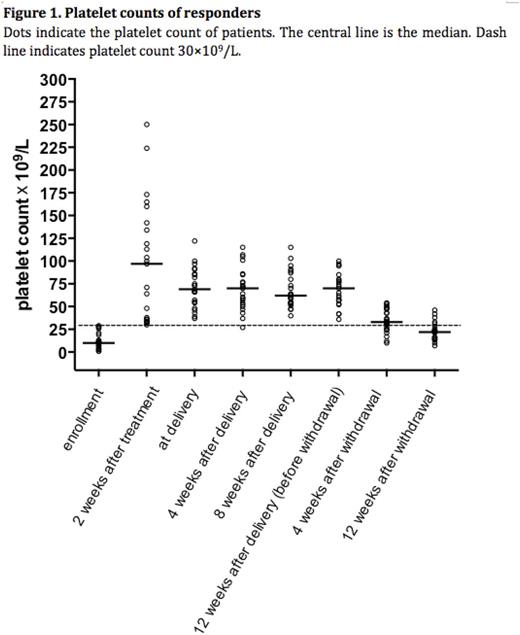
Then the responders received sequential maintenance therapy through the end of week 12 after delivery. To reduce the risk of thrombocytosis during maintenance, dose was tapered to 300U/kg every other day when platelet counts exceeded 50 ×109/L and treatment stopped when platelet counts above 100×109/L. Only one responder had a transient loss of response due to influenza. After dose adjustment of rhTPO from 300U/kg every other day to 300U/kg every day, the platelet count exceeded 50×109/L in the next visit. The platelet counts gradually dropped after withdrawal of rhTPO. The relapse free survival (platelet count at least 30×109/L) at week 4 and week 12 after withdrawal of rhTPO was 69.6%(16/23) and 21.7%(5/23), respectively (Figure 1). Safety and adverse events were evaluated in all 31 participants. rhTPO was well tolerated. Only mild previously reported adverse events were observed, including one case of dizziness, one of fatigue and one of pain at injection site. There were no new reported adverse events during the observation period and no adverse event-related study withdrawals.
In all the 31 newborns, the median age of gestation was 39 weeks (range 36-40, 3 cases had age of gestation< 37 weeks); median birth weight was 3.1 kg (range 2.3-4.2kg, 2 had birth weight<2.5 kg) and the median platelet count at birth was 132×109/L (range 53-263 ×109/L, 9 with platelet<100×109/L) (Table 1). The incidence of premature labor, birth weight and platelet count were similar to previous study on pregnancy outcome in patients with ITP. None of the neonates had bleeding at birth. All 31 infants were evaluated for safety. No adverse effect on the development of newborns was observed during 6 months follow-up. One case of abdominal distension had been reported, which was supposed not associated with rhTPO.
This study demonstrates that rhTPO is a potentially effective, safe and fast-onset treatment for ITP in pregnancy refractory to fist-line therapy and platelet transfusion. Our study paved the way for the clinical application of rhTPO and other thrombopoietic agents in the management of ITP in pregnancy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal