Abstract
In erythroid cells, more than 90% of transferrin-derived iron enters mitochondria where ferrochelatase inserts Fe2+ into protoporphyrin IX. However, the path of iron from endosomes to mitochondrial ferrochelatase remains elusive. The prevailing opinion is that, after its export from endosomes, the redox-active metal spreads into the cytosol and mysteriously finds its way into mitochondria through passive diffusion. An opposing view is that the highly efficient transport of iron toward ferrochelatase in erythroid cells requires a direct interaction between transferrin-endosomes and mitochondria ("kiss-and-run" hypothesis; Ponka Blood 89:1, 1997). Despite the longevity of the prevailing opinion, experimental evidence (Zhang et al. Blood 105:368, 2005; Sheftel et al. Blood 110: 125, 2007) only supports the latter hypothesis.
Using 3D live confocal imaging of reticulocytes following their incubation with MitoTracker Deep Red (MTDR) and Alexa Green Transferrin (AGTf), we have demonstrated transient endosome-mitochondria interactions. We have also documented these interactions by a novel method exploiting flow sub-cytometry to analyze reticulocyte lysates labeled with MTDR and AGTf. We have thusly identified a population of particles labeled with both fluorescent markers, representing endosomes interacting with mitochondria. FACS followed by 2D confocal microscopy confirmed the association of both organelles in the double-labeled population.
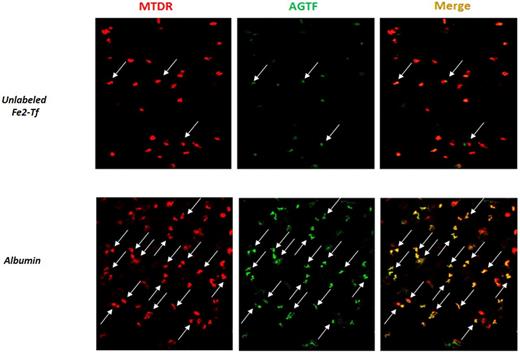
In the current study, we examined whether reticulocyte mitochondria interact with transferrin (Tf) in a cell-free system. Lysates of reticulocytes previously labeled with MTDR were incubated with AGTf for various time intervals. Examination of lysates by 2D confocal microscopy revealed a time-dependent increase in the number of mitochondria in contact with fluorescent Tf. This can be prevented by the presence of excess, unlabeled Fe2-Tf, but not by albumin (Fig.1). Moreover, the addition of unlabeled Fe2-Tf to reticulocyte lysates removed AGTf from mitochondria, indicating that mitochondria from reticulocyte lysates are associated with TfR that can reversibly bind Tf.
In addition, we demonstrate that endosomes containing mutated recombinant holotransferrin, which cannot release iron, remain associated with mitochondria, while endosomes containing mutated recombinant apotransferrin, which cannot bind iron, are not associated with mitochondria. Our findings indicate that endosomes containing holo-Tf promote their attachment to, and drive the detachment of apo-Tf-endosomes from, mitochondria, respectively.
By co-immunoprecipitation assay (from murine eryhroleukemia [MEL] cells and reticulocytes lysates), we purified the voltage-dependent anion channel 2 (VDAC2), which is located at the outer membrane of the mitochondrion (Graham, et al. Curr Top Dev Biol. 59: 87, 2004) with DMT1. We confirmed the colocalization of VDAC2 and DMT1 in MEL cells and reticulocytes by both immunofluorescence and confocal microscopy. Moreover, we found a significant decrease in the number of mitochondria in contact with Tf-endosomes after depletion of VDAC2 in MEL cells or after treatment of reticulocyte lysates with the mitochondrial uncoupler CCCP, further supporting the concept of a physical interaction between endosomes and mitochondria.
To examine a possible role of DMT1-VDAC2 interactions in iron trafficking, we depleted MEL cells of VDAC2 or inhibited VADC2 using erastin (a specific VDAC2 inhibitor that alters its gating) followed by the measurement of 59Fe incorporation from 59Fe-Tf into heme. Our finding of decreased 59Fe incorporation into heme of MEL cells with silenced or inhibited VDAC2 supports the idea that this outer-membrane mitochondrial protein is involved in the interaction of endosomes with mitochondria.
We are currently continuing to delineate the molecular mechanisms involved in endosome-mitochondria interactions focusing on the "signal(s)" that direct iron-carrying endosomes towards mitochondria, the players involved in the docking of endosomes to mitochondria and the "signal(s)" that determine the detachment of iron-free endosomes from mitochondria.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal