Abstract
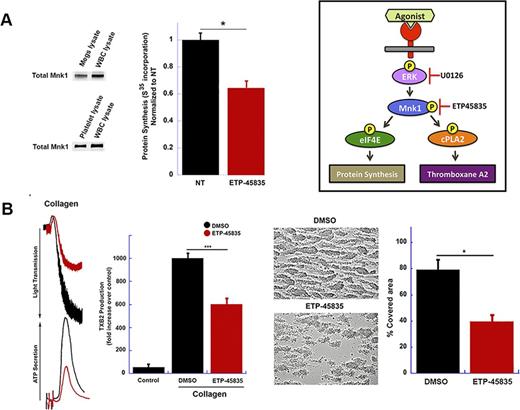
MAPK-interacting kinases (Mnks) are a subfamily of serine/threonine kinases with two isoforms: Mnk1 and Mnk2. Mnks control protein synthesis in neurons and neutrophils, in part through Erk or p38 MAPK phosphorylation. Dysregulated translational control mechanisms are implicated in disorders of cell growth and proliferation. Nevertheless, the expression and function of Mnks in megakaryocytes and platelets is not known. In this study,we investigated the Mnk1 pathway in megakaryocytes and platelets. Mnk1, but not Mnk2, is robustly expressed in human and murine megakaryocytes and platelets (Figure A). Megakaryocytes in their final stages of proplatelet formation expressed activated Mnk1. Interestingly, inhibition of Mnk1 in megakaryocytes with ETP-45835, a specific Mnk1 pharmacological inhibitor, reduced proplatelet formation. Since protein synthesis is critical in the final stages of proplatelet formation, we next sought to determine if Mnk1 regulated translation in megakaryocyte. In neurons, Mnk1 is known to regulate protein translation by releasing CYFIP1 and FMR1 from eIF4E, allowing for the formation of the translational machinery. However, this pathway has not been examined in megakaryocytes. We observed, for the first time, that CYFIP1 and FMR1 are expressed in megakaryocytes and activation of Mnk1 in megakaryocytes resulted in eIF4E phosphorylation and Mnk1-eIF4E complex formation. Importantly, Mnk1 inhibition significantly reduced (~30%, p<0.05) protein synthesis (Figure A). However, the level of inhibition was not as great as with global translation inhibitors, suggesting Mnk1 regulated translation of a subset of mRNAs.
We next sought to determine the role of Mnk1 in circulating platelets. Stimulation of washed platelets with thrombin, collagen, and 2MeS-ADP caused Mnk1 activation in a time-dependent manner. Mnk1 activation was abolished in the presence of U0126, an Erk1/2 specific inhibitor, suggesting that Erk regulates Mnk1 activation in platelets. Inhibiting Mnk1 activation ex vivo with ETP-45385 blocked agonist-dependent platelet aggregation, granule secretion, and thromboxane generation (Figure B). The addition of arachidonic acid to ETP45385-treated platelets fully rescued platelet functional responses, demonstrating that Mnk1 activation in platelets regulates the arachidonic acid pathway. Blocking Mnk1 activation with ETP-45385 also markedly inhibited platelet adhesion to collagen under flow conditions (Figure B). Mnk1 inhibition in vivo with ETP-45385 protected mice from collagen and epinephrine-induced pulmonary embolism.
Taken together, our findings demonstrate that Mnk1 a canonical translation regulatory protein, mediates megakaryocyte proplatelet formation and protein translation, but it also responsible for reducing susceptibility to in vivo thrombosis through thromboxane dependent mechanism. We hypothesize that Mnk1 and its downstream targets represent new regulatory mechanisms for translational control in megakaryocytes and platelets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal