Abstract
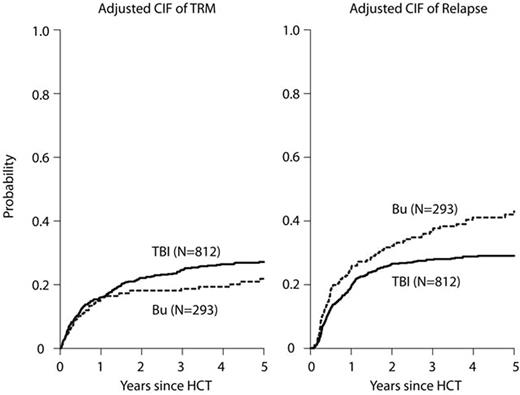
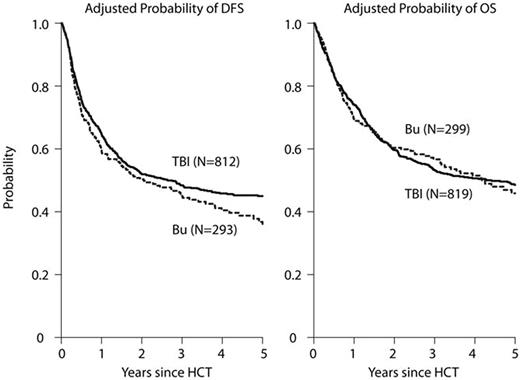
Total body irradiation (TBI)-based conditioning regimens are considered the standard of care for patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic hematopoietic cell transplantation (HCT). However, due to concerns regarding acute and long-term toxicities, non-TBI regimens, commonly including busulfan (BU), have been increasingly explored. We performed a retrospective cohort analysis with the hypothesis that there would be equivalence of these two myeloablative approaches by reviewing outcomes for adult patients (pts), aged 18-60 years, undergoing a first, well-matched sibling, related or unrelated donor HCT in CR1 or CR2 reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 2005-2014. Eight hundred nineteen pts received TBI (63% 9-12 Gy, 37% ≥13 Gy) combined with etoposide (25%) or cyclophosphamide (Cy) (75%) and 299 pts received intravenous BU combined with a second alkylator Cy (15%) or melphalan (Mel) (13%), or a nucleoside analogue fludarabine (Flu) (41%) or clofarabine (Clo)(30%). The majority of the BU-based pts were treated at the Moffitt or MD Anderson Cancer Center. The BU-based regimens were grouped together for analyses since no significant differences in basic outcomes among the different chemotherapy-only regimens were noted. Patients in the BU-containing group were older but had better performance status, took longer to achieve CR1 and longer to receive HCT; more received peripheral blood than marrow grafts, peri-HCT ATG, pre- and post HCT tyrosine kinase inhibitors ( TKI), and were treated more recently than pts in the TBI-based group (Table 1). With median follow-up of 3.6 years for the BU-based group and 5.3 years for the TBI-based group, adjusted 3 year outcomes showed treatment-related mortality (TRM) BU 19% vs. TBI 25% (p=.04); relapse BU 37% vs. TBI 28% (p=.007); disease-free survival (DFS) Bu 45% vs. TBI 48% (p=.35); and overall survival (OS) BU 57% vs. TBI 53% (p=.35) (Figures A-B). Patients in the BU group had significantly more grade II-IV acute GVHD (47% vs. 40%, p=.025), but marginally less chronic GVHD (49% vs. 55% at 3 years, p=.073). In multivariate analysis, the BU group had a significantly higher rate of acute GVHD after day 50 (RR 1.75, 95% CI 1.19-2.58, p=.004), but marginally less chronic GVHD (RR 0.83, 95% CI 0.68-1.01 p=.059) and higher risk of relapse (RR 1.46, 95% CI 1.15-1.85 p=.002) compared with TBI-based regimens. Despite the observed higher risks of acute GVHD and relapse, BU-based conditioning led to similar TRM, OS, and DFS following HCT for ALL.
Ciurea:Spectrum Pharmaceuticals: Other: Advisory Board; Cyto-Sen Therapeutics: Equity Ownership. Seftel:Otsuka: Research Funding. Pulsipher:Medac: Other: Housing support for conference; Chimerix: Consultancy; Jazz Pharmaceutical: Consultancy; Novartis: Consultancy, Other: Study Steering Committee. Champlin:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal