Abstract
Background: DI-Leu16-IL2 is a recombinant fusion immunocytokine composed of interleukin-2 and a CD20-targeting monoclonal antibody that maintains the activities of both antibody and cytokine components but is also involved in tumor targeting, engagement of the immune system, and induction of an anti-cancer vaccine effect.
Methods: In this multicenter open label, dose escalation trial to determine the maximum tolerated dose (MTD), optimal biological dose (OBD), and recommended phase 2 dose (RP2D), we evaluated the safety, tolerability, and efficacy of subcutaneously (SC) administered DI-Leu16-IL2 in patients (pts) with relapsed or refractory CD20+ B-cell lymphoma (NCT01874288). Following peripheral B-cell depletion with low-dose rituximab (50mg/m2), DI-Leu16-IL2 was administered SC for 3 consecutive days every 21 days for 6 cycles. A 3+3 dose escalation trial design began at 0.5 mg/m2. Responses were evaluated by PET in pts who had received at least 2 cycles of therapy. Immunologic correlates were done including peripheral blood flow cytometry and serum cytokine levels. Responding (complete or partial response, CR or PR) and stable disease (SD) pts were permitted to continue treatment for an additional year.
Results: Twenty-two pts in 5 dose cohorts have been enrolled, 3 at 0.5 and 1mg/m2, 7 at 2mg/m2 and 4mg/m2, and 2 at 6mg/m2. The median age is 64 years (40-84), median prior therapies is 4 (range 1-21), with 100% having received prior rituximab and chemotherapy, and 41% with prior autologous stem cell transplant. Dose limiting toxicities (DLT) occurred in 2 pts at 6 mg/m2, both with delayed (8-10 days) grade 3 cytokine release syndrome (CRS), suggesting effector cell expansion as the cause. None of the CRS required treatment with tocilizumab, however one required dose reduction, and the other discontinued therapy.
The Gr 3-4 drug-related adverse events (AEs) that required dose reduction were: QTC prolongation (Gr 3) in one patient at 4 mg/m2, tumor lysis syndrome (Gr 3) at 4mg/m2, and diarrhea (Gr 3) at 1mg/m2. Hematologic AEs were eosinophilia (Gr 1-2 = 22.7%), anemia (Gr 1-2 = 45%, Gr 3-4 = 9%), thrombocytopenia (Gr 1-2 = 36%, Gr 3-4 = 4.5%), neutropenia (Gr 1-2 = 14%, Gr 3-4 = 14%) in the 22 pts evaluable for toxicity. Other AEs were local injection site reactions (Gr 1-2 = 82%), pruritus (Gr 1-2 = 82%), edema (Gr 1-2 = 23% Gr 3-4 = 5%), fever (Gr 1-2 = 41%, Gr 3-4 = 5%), and chills (Gr 1-2 = 59%). Six pts have been enrolled at 4mg/m2 and no DLTs have been encountered. The MTD was determined to be 4mg/m2.
Patients with CR or PR had higher pre-treatment absolute lymphocyte counts (>700/mcL) than pts with progressive disease (P < 0.05). Repetitive SC dosing elicited lymphocyte expansion at all dose levels. Although T-regulatory cell expansion was observed in the blood, it was not associated with a worse response. Pts achieving CR showed a trend towards lower % of granulocyte-like myeloid derived suppressor cells. Pts showed reproducible increases in cytokine levels of IL-10, MCP-1, IFNgamma, and TNFalpha. Pharmacokinetic data showed consistent drug levels between cycle 1 and 2.Therefore, the OBD and RP2D was determined to be 2mg/m2 which produced 3 CR and 3 SD.
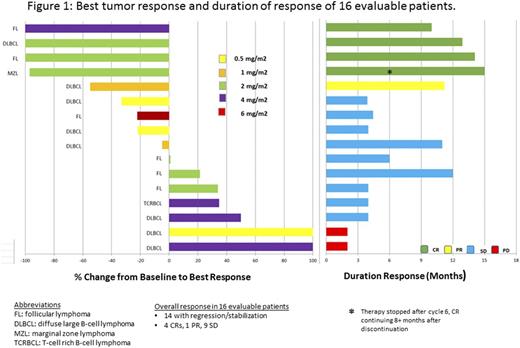
Durable tumor regression or stabilization was noted in 14 of 16 (88%) evaluable pts. There were 4 CR: 1 diffuse large B-cell lymphoma (DLBCL), 2 follicular lymphoma and 1 marginal zone lymphoma; 1 PR (DLBCL) and 9 SD, and objective response rate of 31% [Figure 1]. The median duration of disease stabilization was 8 months (4-14). Of the responders, the median duration of response was 13 months (10-15). One CR continues 11 months after stopping therapy at cycle 6.
Conclusions: Promising clinical efficacy of DI-Leu16-IL2 has been observed with long-term responses in relapsed/refractory B-cell NHL pts. SC administration has permitted higher doses than could be achieved with IV treatment, however, at the higher dose range, CRS was observed. Even though the MTD was 4mg/m2, given the responses, AEs, and biologic activity in the Phase I study, the RP2D was determined to be 2mg/m2. Responses were associated with a higher starting ALC, and lymphocyte expansion was dose dependent, with reproducible serum cytokine profiles. The protocol continues to enroll pts in the expansion cohorts of this phase I/II study. DI-Leu16-IL2 given subcutaneously is safe and effective, and induces durable responses even after discontinuation of therapy, suggesting an anti-tumor vaccination effect.
Lansigan:Celgene: Consultancy; Teva: Research Funding; Pharmacyclics: Consultancy; Spectrum: Consultancy, Research Funding. Vlock:Alopexx Oncology, LLC: Employment. Gillies:Provenance Bioparmaceuticals Corp: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal