Abstract
Introduction: Currently available therapy options for primary mediastinal large B-cell lymphoma (PMBCL) generally yield poor treatment outcomes. Like classical Hodgkin lymphoma (cHL), PMBCL frequently exhibits 9p24.1/PD-L1/PD-L2 copy number alterations and rearrangements and associated PD-L1 and/or PD-L2 overexpression, which may facilitate immune evasion. The genetics of PMBCL could thus make it susceptible to PD-1 blockade. KEYNOTE-013 (NCT01953692) is an ongoing multicenter, multicohort Phase 1b trial evaluating safety, tolerability, and antitumor activity of pembrolizumab, a humanized anti-PD-1 monoclonal antibody, in patients with hematologic malignancies. Here we report results from the first 19 patients enrolled in the PMBCL cohort of KEYNOTE-013, with a follow-up of up to 2 years.
Methods: This independent cohort of KEYNOTE-013 is enrolling patients with relapsed/refractory (R/R) PMBCL who have relapsed after or are ineligible for autologous stem cell transplant (SCT). Patients received pembrolizumab IV 10 mg/kg every 2 weeks (Q2W), which was later changed by protocol amendment to a fixed dose of 200 mg every 3 weeks (Q3W), following PK/PD studies demonstrating both regimens to be equivalent. Treatment continues for up to 2 years or until unacceptable toxicity or confirmed disease progression. Treatment response is evaluated using IHP 2007 criteria by positron emission tomography and computed tomography at weeks 6 and 12, and every 9 weeks thereafter. Primary end points are safety and objective response rate (ORR). Secondary end points include complete remission (CR) rate and duration of response (DOR). The safety population consists of all patients who receive ≥1 dose of study drug and the efficacy population of all patients who progress prior to or reach the first efficacy evaluation. Whole blood was collected at predefined time points before and during pembrolizumab treatment for RNA and DNA extraction and biomarker analysis using a number of genomic-based assays, including NanoString and RNA sequencing.
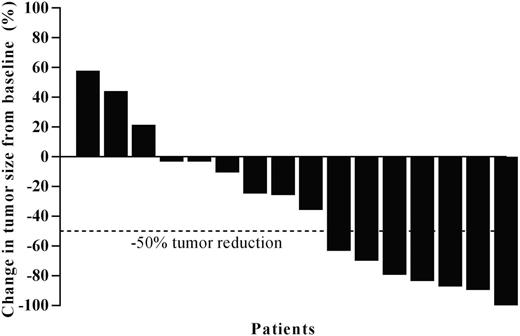
Results: As of the analysis cutoff date (May 27, 2016), 19 patients were enrolled in the PMBCL cohort, 18 were treated, and 16 had ≥1 post-baseline efficacy evaluation. The first 11 patients were to receive pembrolizumab IV 10 mg/kg Q2W (1 was not treated due to early progressive disease); all subsequent patients received 200 mg Q3W. Median age was 30.5 years (range, 22-62). Most patients (72%) were female. 61% of patients had ≥3 prior lines of therapy, 33% had prior autologous SCT, and 61% prior radiation. In the efficacy population, 16 patients were evaluable for response: one discontinued treatment based on clinical progression before the first response assessment (this patient was considered a nonresponder), the other had not reached the first assessment.The ORR was 41% (7/17), with 2 patients achieving a CR and 5 patients a partial response; 35% (6/17) had stable disease as best response. Overall, 81% (13/16) of evaluable patients had target lesion reductions (Figure). With a median follow-up duration of 11.3 months (range, 3.4 to 27.4 months), median DOR was not reached, and there were 6 ongoing responses at time of current data cutoff. DOR ranged from 2.4+ to 22.5+ months; DOR in the 2 patients with CR was 2.4+ and 20.5+ months. Two patients received an allogeneic SCT: one had SD, the other PD on pembrolizumab. Ten patients discontinued treatment: 5 due to progressive disease based on imaging, 4 for clinical progression, and 1 due to physician decision. Two patients reached the maximum 2 years of treatment and remain in remission. Six patients experienced serious AEs, and none discontinued due to AEs. Eleven patients (61%) experienced treatment-related adverse events (TRAEs), mostly grade 1-2. One patient experienced a TRAE of grade 3 neutropenia and another a TRAE of grade 4 venoocclusive liver disease (VOD; after allogeneic SCT during the follow-up period after pembrolizumab was discontinued), the only serious TRAE. The patient recovered from the VOD. There were no treatment-related deaths.
Conclusions: These results from an on-going study in heavily pretreated R/R PMBCL patients demonstrate that PD-1 blockade with pembrolizumab has a manageable safety profile and promising antitumor activity. Due to these results, a pivotal global multi-center Phase 2 trial, KEYNOTE-170, is further evaluating single agent pembrolizumab in patients with R/R PMBCL.
Zinzani:MorphoSys: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Ribrag:Esai: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; ArgenX: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; NanoString: Membership on an entity's Board of Directors or advisory committees. Moskowitz:Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Celgene Corporation: Consultancy; Genentech BioOncology: Consultancy. Michot:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Kuruvilla:Merck & Co., Inc.: Consultancy, Honoraria. Balakumaran:Merck & Co.: Employment, Other: stock, stock options. Zhang:Merck & Co., Inc.: Employment, Other: stock, stock options. Marinello:Merck & Co., Inc.: Employment, Other: stock, stock options. Chlosta:Merck & Co., Inc.: Employment, Other: stock, stock options. Gustafson:Merck & Co., Inc.: Employment, Other: stock, stock options. Shipp:Merck & Co., Inc.: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Armand:Otsuka: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Infinity: Consultancy; Roche: Research Funding; Sequenta: Research Funding; Tensha: Research Funding; Sigma Tau: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal