Abstract
Introduction: CD19-targeted chimeric antigen receptor-modified (CAR) T cells have demonstrated considerable therapeutic efficacy in patients (pts) with relapsed and/or refractory (R/R) B cell ALL (B-ALL), resulting in rapid and often durable complete responses (CR). In contrast, a smaller subset of pts with R/R CLL have achieved CR following CD19-targeted CAR T cell therapy. Ibrutinib (IBR), which has considerable efficacy as a single agent in pts with R/R CLL, may modulate antitumor T cell immune responses. Others have observed enhanced ex vivo expansion of autologous T cells collected from pts with IBR exposure in response to CD3/CD28 bead stimulation, and improved CD19-targeted CAR T cell engraftment and antitumor efficacy in human xenograft models (Fraietta et al., Blood, 2016). Herein, we report on adults with CLL treated with IBR at the time of autologous T cell collection and/or around the time of CAR T cell infusion enrolled in our phase I clinical trial of CD19-targeted CAR T cells for adults with R/R CLL or B-cell NHL (NCT00466531).
Methods: Eligible pts underwent leukapheresis and T cells were transduced with a retroviral vector encoding a CAR comprising a CD19-specific scFv and CD28 and CD3ζ signaling domains (19-28z). The present analysis is limited to pts with CLL. We identified pts with CLL treated with IBR at the time of leukapheresis and/or around the time of conditioning chemotherapy (CCT) and CAR T cell infusion. As a control group, we additionally identified all evaluable IBR-naïve pts with CLL treated on this study. Response was assessed by NCI-WG criteria. Cytokine levels were measured prospectively before and after CCT and CAR T cell infusion.
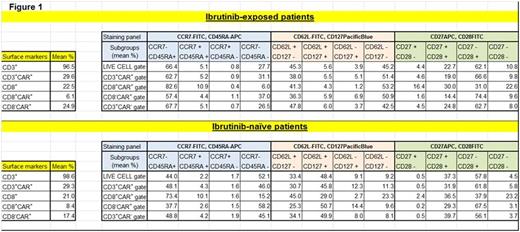
Results: 5 pts (male, n=3), median age 58 at CAR T cell infusion (range, 43-66) with R/R CLL (TP53 loss, n=2) underwent therapy with IBR at leukapheresis (n=4) and/or immediately prior to or through CCT (cyclophosphamide [Cy], n=2; fludarabine [Flu]+Cy, n=3) and CAR T cell infusion (n=5). 6 additional evaluable pts with R/R CLL remained IBR-naïve through CCT (Cy, n=4; bendamustine, n=2) and CAR T cell infusion. A non-significant trend toward greater median cumulative fold T cell expansion ex vivo was noted in the 4 pts on IBR (vs the 7 not on IBR) at leukapheresis (374 [171-1518] vs 160 [49-468], p=0.13), with similar median manufacturing time (13.5 vs 15 days). End of process (EOP) T cells in pts undergoing collection while on IBR (vs those not on IBR) demonstrated a greater fraction of CD8+CAR+ T cells with a CD62L+CD127+ (central memory) phenotype (mean 29.0 vs 4.3%, p=0.10) and decreased fraction of CD62L- T cells (effector/effector memory phenotype) across CD8+CAR+ (mean 26.5 vs 54.4%, p=0.06) and CD4+CAR+ (mean 24.0 vs 57.8%, p=0.03) T cell subsets (Fig 1).
IBR-treated pts received median 1x107 19-28z+ CAR T cells/kg (3x106-3x107/kg) and IBR-naïve pts received median 1x107 19-28z+ CAR T cells/kg (6x106-4x107/kg). Fevers developed in all 11 pts and began on the first day of infusion in 4/5 IBR-treated pts (vs 2/6 IBR-naïve pts); 2/5 IBR-treated pts (vs 0/6 IBR- naïve pts) developed severe CRS and required vasopressors for hypotension in addition to tocilizumab. IBR-treated pts additionally exhibited greater median peak levels of multiple immunoregulatory cytokines associated with CRS, including IL-6, IL-10, IL-2, IL-5, IFN-γ, FLT3L, fractalkine, and GM-CSF.
In total, 5 of 11 enrolled pts with CLL (45%) treated with CCT and 19-28z CAR T cells achieved objective response (minimal residual disease [MRD]- CR, n=2; maintenance of MRD+ CR, n=1; PR, n=2); ORR was 4/5 among IBR-treated pts (1 MRD- CR, 1 MRD+ CR, 2 PR; p=0.08 for ORR between IBR-treated vs IBR-naïve pts). 2 pts remain in MRD- CR at 16 and 50 months. Maximal CAR T cell persistence observed to date is 159 days; peak vector copy levels by qPCR were highest in the 2 pts attaining MRD-negative CR.
Conclusions: Prior therapy with IBR may influence EOP CAR T cell phenotypes. Prior ± concurrent IBR may improve antitumor responses following 19-28z CAR T cell administration, though small numbers of pts and differences in CCT regimens limit firm conclusions based on these data. Additionally, prior ± concurrent IBR may amplify CRS, though more intensive CCT (e.g. Flu/Cy vs Cy) may also enhance CAR T cell expansion in vivo and intensify CRS. Further strategies to overcome the inhibitory microenvironment and enhance CAR T cell expansion and efficacy in pts with R/R CLL are in preparation.
Park:Amgen: Consultancy; Genentech/Roche: Research Funding; Juno Therapeutics: Consultancy, Research Funding. Riviere:Juno Therapeutics: Consultancy, Equity Ownership, Patents & Royalties, Research Funding. Sadelain:Juno Therapeutics: Consultancy, Equity Ownership, Patents & Royalties. Brentjens:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal