Abstract
Steady state erythropoiesis is regulated by interaction of EPO with its receptor, EPO-R which activates Ca2+signaling and terminal maturation [Miller, Blood 1989;73:1188]. Under stress, GR switches EPO-R signaling to a proliferation mode allowing erythroblast (Ery) amplification [Zhang, Nature 2013;499:92]. However, for recovery from anemia to occur, a mechanism, still to be identified, must counteract GR and switch EPO-R signaling back to a maturation mode.
CALR chaperones other proteins to their active sites[Michalak, Biochem J 1999;344: 281]. By importing GR from the nucleus, CALR resets the stress response of murine fibroblasts. This function is regulated by the exposure of its C-terminal domain (C-CALR) sustained by Ca2+ [Holaska, Mol Cell Biol 2002;22:6286]. Whether CALR regulates nuclear import of GR in human Ery and how this regulation is modulated by Ca2+and EPO is unknown. Studies were performed to fill this gap.
By WB, human Ery expanded in vitro from adult blood expressed robust levels of CALR, detectable with N-CALR- and C-CALR antibodies, and of GR. By flow cytometry, N-CALR and C-CALR were detectable on the surface of cells from the proerythroblast (proEry) to polychromatic stage. Expression of N-CALR, the epitope most exposed on the surface, was up-regulated by EPO. By confocal microscopy, both N-CALR and C-CALR were detected in non-permeabilized Ery but only C-CALR was detectable in cells permeabilized to reveal their cytoplasmic content. Intra-cytoplasmic C-CALR staining was robust in proEry, barely detectable in orthochromatic cells and enhanced by adding Ca2+ to the permeabilization buffer. Double staining of C-CALR with GR or LaminB1 (an inner nuclear membrane protein) indicated that C-CALR/GR were associated in the perinuclear area of the cytoplasm but that C-CALR remained distinct from Lamin B1, suggesting that C-CALR/GR co-localize at the cytoplasm/nuclear boundary. These results suggest that C-CALR mediates nuclear export of GR and that this function is regulated by Ca2+.
This hypothesis was tested first by determining content and localization of GRα and C-CALR in nuclear and cytoplasmic fractions of Ery treated with Dex, EPO and SCF by WB. Although none of these stimulations altered the total content of these proteins, some of them affected their compartmentalization: Dex rapidly (15') induced GR S211 phosphorylation and increased the levels of GRα in nuclear fractions. These effects were antagonized by addition of the GR inhibitor RU486. The nuclear levels of GRα were modestly increased also by SCF but strongly decreased by EPO. By contrast, regardless of the stimuli, C-CALR was detected mostly in cytoplasmic fractions.
The effects of these stimulations were also assessed by confocal microscopy. Exposure to Dex and EPO increased by 15' but decreased by 4h the nuclear localization of GR and significantly increased the levels of C-CALR and of C-CALR/GR co-localization detected in the cytoplasm by 4h, suggesting that C-CALR drives nuclear export of GR. In agreement with this hypothesis, multi-regression analyses comparing single C-CALR and total and cytoplasmic GR signals as independent parameters against merged signals as dependent parameter in 410 individual proEry indicated that the levels of C-CALR/GR co-localization directly correlated with the cytoplasmic levels of both C-CALR and GR but that the greatest p-values were observed for C-CALR (p=0.0000 vs 0.045). Moreover, treatment with the nuclear export inhibitor Leptomycin greatly decreased C-CALR/GR co-localization, increased nuclear localization of GR in proEry, both in untreated and EPO-treated cells, and reduced Ery proliferation in response to Dex down to levels sustained by Dex with RU486.
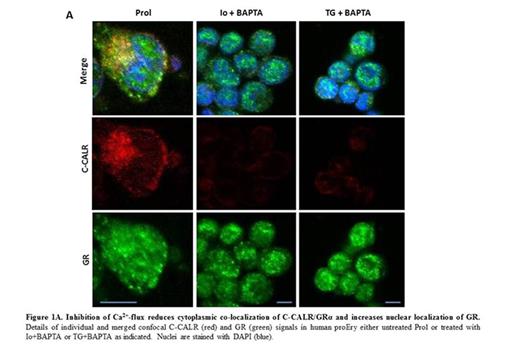
To assess the role of Ca2+ in the nuclear export activity of C-CALR confocal microscopy and proliferation studies on Ery exposed or not to the Ca2+ chelator BAPTA in combination with Ionomycin (Io, a raiser of intracellular Ca2+) or Thapsigargin (TG, a reducer of Ca2+in endoplasmic reticulum) were performed. These treatments greatly decreased C-CALR and C-CALR/GR co-localization in the cytoplasm, increased GR signals in nuclei and reduced the proliferation of Ery in response to Dex.
These results suggest that conformation changes of C-CALR induced by Ca2+signaling downstream to EPO-R sustain nuclear export of GR, resetting the stress-response of normal proEry allowing them to undergo terminal maturation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal