Abstract
Introduction: Early reduction of BCR-ABL transcript level has been associated with improved outcomes in CML treatment. Inability to achieve early molecular response(MR) at 3 months (M3>10%) is considered a predictor factor for unfavourable outcome. However, the kinetics of BCR-ABL transcript level reduction measured at early time points have shown to be an independent predictor of response.The aim of this analysis was to determine whether the "M3-M6" status is critical to categorize CML patients (pts) focusing in high-risk group.
Method: Molecular monitoring was performed in all pts prior treatment (M0), at months 3 (M3), 6 (M6), 12 (M12) and every 6 months thereafter, applying Q-PCR method according international recommendations. Results of BCR-ABL1 transcript level were reported on the international scale as IS-BCR-ABL %. Optimal responses: M3≤10%, M6≤1%, M12≤0,1%. Deep responses (MR4.0): ≤0,01% or undetectable/10.000 ABL copies.
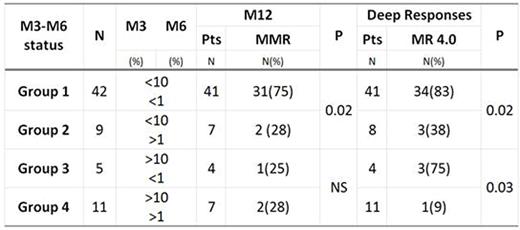
Results: A total of 70 CML pts were included, median age 49 (19-82), female 39%. First line treatment: sustained branded 81% and generic 19% TKIs. Imatinib 59%, Dasatinib and Nilotinib 41%. Sokal risk score: low (L) 51%, intermediate (In) and high (H) 49%. Optimal responses at molecular milestones: 75% at M3, 72% at M6, 61% at M12 and 53% pts achieved MR4.0. Event-free survival (EFS) was evaluated according to time point M3: M3≤10% group had significantly better EFS compared with the M3>10% (96% vs 70%; P=0.028). M3-M6 status defined 4 groups of pts: M3≤10%-M6≤1%, M3≤10%-M6>1%, M3>10%-M6≤1%, M3>10%-M6>1%. Molecular response evolution by M3-M6 status is described in Table 1. EFS stratified by groups according to combined M3-M6 responses showed significant differences: 92% for group 1, 87% for group 2, 68% for group 3, 54% for group 4. (P=0.002). M6 time point was shown to be critical in 32 high-risk pts (H+In): 17 pts with M6 ≤1% showed significant differences in MR4.0 achievement compared to 15 pts with M6 >1% (82% vs 27% P=0.02). Better EFS was observed in this high-risk group under branded vs generic TKIs treatment (97% vs 54% P=0.04). Statistical differences in deep responses and MMR at M12 were observed between branded and generic TKIs independently of Sokal risk (P=0.06, P=0.02).
Conclusions: M3≤10% pts showed a favourable evolution with better EFS than M3>10% group. However not all patients with M3<10% had a similar behavior, showing lower rates of MMR at M12 and deep responses in those pts with M6>1%. In pts with M3 >10% and optimal response at M6 also showed higher MR4.0 rate. Our study supports that M6 is a crucial endpoint to predict MMR at M12 and deep responses in CML pts.Pts with M3≤10% without optimal response at M6 (>1%) had a worse evolution than those slow responders who showed M3>10% and M6≤1%.High-risk pts are still a challenge, observing better outcomes in those under branded TKIs treatment. The M3-M6 status would be a prognostic marker of responses and EFS in chronic phase CML pts treated with TKIs. Our data support the critical role of M6 response in non-optimal M3>10% pts and intermediate and high risk Sokal score. Treatment adherence is mandatory for achieving and sustaining optimal responses. This multicentric Argentine study, reinforces the importance of clinical follow-up and molecular monitoring under IS standardization at early time points. Education on early molecular monitoring with adequate resources must continue to be an objective in our region.
Pavlovsky:Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau. Moiraghi:Novartis: Speakers Bureau; Bristol: Speakers Bureau. Varela:Novartis: Speakers Bureau; Bristol: Speakers Bureau. Enrico:Novartis: Honoraria, Patents & Royalties; Bristol Myers squib: Speakers Bureau. Brodsky:International PNH Registry: Other: -; Alexion Pharma Argentina: Speakers Bureau. Pavlovsky:Novartis: Speakers Bureau; Janssen: Speakers Bureau; Bristol Myers Squib: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal