Abstract
Introduction: The introduction of imatinib mesylate as first-line treatment has led to great improvements in the prognosis of patients with chronic myeloid leukemia (CML). The most used prognostic scores are Sokal, EUTOS and Hasford. Also, the time from diagnosis to imatinib and cytogenetic response influence the prognosis of patients with CML. However, there is no information about CML patients in chronic phase treated with imatinib in developing countries.
Objectives: The aim of this study was to determine the prognostic factors in Peruvian patients with CML in chronic phase treated with imatinib (Glivec).
Methods: Retrospective cohort of peruvian patients with chronic phase CML that received Imatinib (Glivec-Novartis) through the Glivec International Patient Assistance Program (GIPAP) in a national cancer center. The patients were classified according to prognostic scores of Sokal, EUTOS and Hasford at the beginning of imatinib therapy. The overall survival (OS) was calculated from the date of initiation of imatinib until the date of death or last contact. Event-free survival (EFS) was calculated from the date of start of treatment to date of occurrence of death from any cause during treatment, progression to accelerated phase or blast crisis CML or loss of major cytogenetic response. Transformation-free survival (TFS) was calculated from the date of start of treatment to date of occurrence of accelerated progression to blastic phase. Survival curves were estimated by Kaplan-Meier and comparison was done by log-rank test. Multivariate analysis for OS was performed with the Cox proportional hazard regression model.
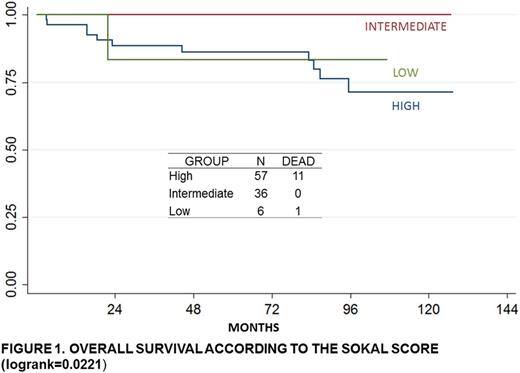
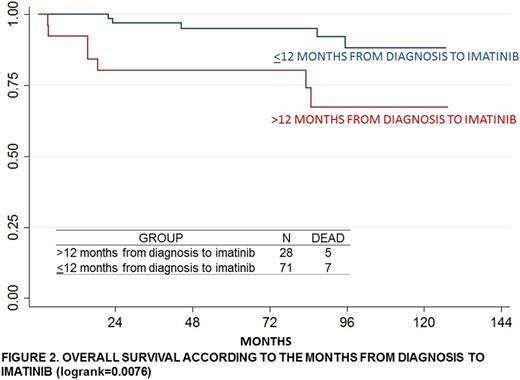
Results: 99 patients met the eligibility criteria and had a median observation time of 71.3 months. The average time from diagnosis to start imatinib was 11.4 months (CI 7.58-15.31). 14% of the patients had an age>55 years. The complete cytogenetic response at 12+3 months was 36%; the partial response was 16.67% and the lower response was 13.89%. According to the criteria of Sokal, EUTOS and Hasford, 59.58%, 49.49% and 27.27% were classified as higher risk, respectively. The 5-yrs-OS was 66.67% (CI 0.04-0.18%); 5-yrs-EFS, 68.63% (CI 0.13-0.38%) and 5-yrs-TFS, 60.82% (CI 0.21-0.41%). The patients with an age>55 years had significantly lower OS compared with the younger patients (p=0.039). The patients with a time higher than 12 months from diagnosis to imatinib had significantly lower OS than the patients that started it earlier (p=0.0076). The OS was significantly different according to the risk groups of Sokal score (p=0.0221). Also, significant difference was found in the EFS according to cytogenetic response (p=0.042). In the multivariate analysis, the factors that influence OS were high risk according toSokal score (HR=9.42 CI1.09-81.29) and a time greater than 12 months from diagnosis Imatinib (HC=3.53 CI1.12-11.18).
Conclusion: In this Peruvian population, the majority of patients are at high risk groups of Sokal, EUTOS and Hasford scores. High risk according to Sokal score and a time higher than 12 months from diagnosis to imatinib were the main prognostic factors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal