Abstract
Molecular tests are the best way to monitor CML course in patients under treatment with tyrosine kinase inhibitors (TKI). International guidelines indicate the absolute copy number of the control gene ABL1 as reference for the definition of the sensitivity of the analytical method. A general implementation of the International Guidelines (EUTOS) and the moving forward of current technologies towards one-step reactions, that allow direct testing from the patient RNA, require continuous verification of the method performances.
Here, as Italian laboratory network for the standardization of CML diagnosis (LabNet), we performed a comparative study across the three reference laboratories in order to evaluate the inclusion of "BCR-ABL P210 ELITe MGB® Kit" (ELITechGroup S.p.A.) one-step assay among the technologies indicated in the Laboratory Recommendations and Indications (R.I.L.) of the Italian Network for CML monitoring. "BCR-ABL P210 ELITe MGB® Kit" is a new assay that allows to perform in a unique reaction the retro-transcription and the amplification of the extracted RNA sample.
In this study 30 RNA extracted from whole blood samples of CML patients at different stages of the disease and centrally distributed to the other reference labs have been analyzed. All laboratories tested 300 ng per reaction of each RNA according the one-step approach and the same RNA according each own routine method. Moreover, in the same experiments, the European Reference Material certified plasmid ERM-AD623 has been evaluated.
Our results show an increased analytical sensitivity in detection of both genes (BCR/ABL1 and ABL1): the limit of detection of the one-step reaction is as low as 0.001% IS BCR/ABL1. By testing the ERM-AD623 at 1 copy/reaction the rate of PCR positivities is 63%, and the average estimated quantity is 2.5 (SD = ± 1.5) copies/reaction.
The linear measurement range of BCR/ABL1 and of the control gene ABL1 evaluated using the ERM-AD623 reference material are linear and equivalent in the range of 102-107 copies/reaction. Quantifications obtained with this kit are aligned to the European Reference Material.
Using 7500 Fast Dx Real-Time PCR Instrument or 7900 Real-Time PCR System (Applied Biosystem, Thermo Fisher Scientific), we confirm that the calibrator of the "BCR-ABL P210 ELITe MGB® Kit" is aligned to the ERM-AD623 DNA international standard and we demonstrated the inter-laboratory low variability and good linearity of the method by processing the secondary reference material aligned to WHO primary reference material.
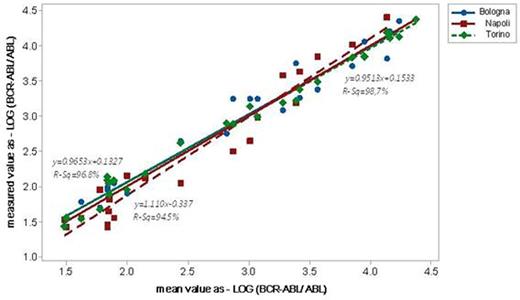
By analysis of 30 RNA of CML patients we observed high results reproducibility among laboratories (figure 1).
In addition, at comparison with the individual routine methods (ipsogen BCR-ABL1 Mbcr IS-MMR DX, P210 PHILADELPHIA Q-PCR Alert kit. and an home-made assay) we report up to 97.4% correlation of BCR-ABL P210 ELITe MGB® kit results.
In conclusion, our data demonstrate that "BCR-ABL P210 ELITe MGB® Kit" is a rapid, reproducible assay, aligned and calibrated towards the current goal standards BCR/ABL1 assays. It allows direct testing from RNA samples while maintaining the desired sensitivity. By requiring reduced hands on time of the operators and by allowing direct testing of RNA, "BCR-ABL P210 ELITe MGB® kit" will provide a significant improvement in the standardization of the molecular approach to CML monitoring.
BCR-ABL P210 ELITe MGB® Kit reproducibility with clinical samples. Data of the individual laboratory were plotted against the mean assigned value. The regression fit of all data is R-Sq=96.8%.
BCR-ABL P210 ELITe MGB® Kit reproducibility with clinical samples. Data of the individual laboratory were plotted against the mean assigned value. The regression fit of all data is R-Sq=96.8%.
Castagnetti:Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; ARIAD Pharmaceuticals: Consultancy, Honoraria. Martinelli:ARIAD: Consultancy; Genentech: Consultancy; Roche: Consultancy; Amgen: Consultancy; MSD: Consultancy; Pfizer: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Genentech: Consultancy; Amgen: Consultancy; Novartis: Speakers Bureau; MSD: Consultancy; Roche: Consultancy; ARIAD: Consultancy; Pfizer: Consultancy, Speakers Bureau. Saglio:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal