Abstract
Background: Concerns have been raised whether immune checkpoint inhibitor therapy in the alloBMT setting will result in graft versus host disease (GvHD) and transplant related mortality (TRM). We report our experience with a variety of checkpoint inhibitors used before or after allogeneic bone marrow transplantation (alloBMT). Our series comprises patients who received T cell-replete hematopoietic stem cells from HLA-haploidentical or -matched donors and is limited to those treated with post-transplant cyclophosphamide (PTCy) as primary GvHD prophylaxis.
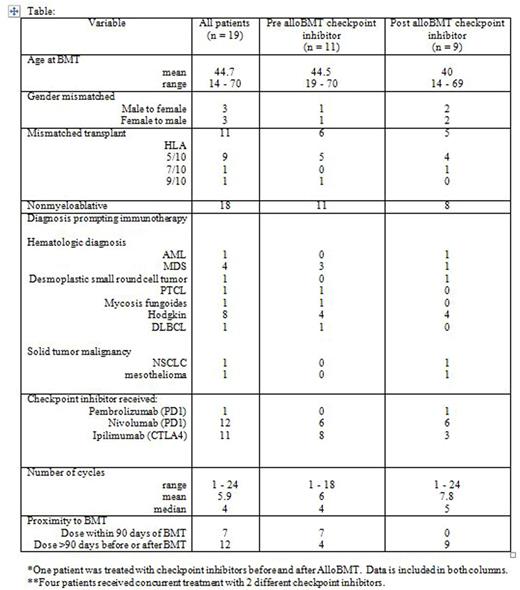
Patient selection: We retrospectively reviewed the records of alloBMT recipients who received PTCy and received checkpoint inhibitor therapy before or after alloBMT. GvHD was assessed using the CIBMTR GVHD index.
Results:
Eleven patients received checkpoint inhibitor therapy prior to alloBMT: anti-PD-1: Nivolumab n=6, anti-CTLA4: Ipilimumab n=8 (3 patients received both nivolumab and ipilimumab). These patients received a median of 4 (range 1 - 18) cycles of therapy. The median interval from last checkpoint inhibitor treatment to day of transplant was 43 (range 18-302) days. All patients received nonmyeloablative conditioning; 6 received partially mismatched allografts (5 were HLA haploidentical). Four patients developed Grade II aGvHD: Three patients who had received partially mismatched allografts (haplo-2, 9/10 unrelated-1) experienced stage 3 cutaneous GvHD only; one patient who received a 10/10 unrelated donor allograft developed stage 3 cutaneous GvHD with stage 1 liver involvement. Three patients were on immunosuppression when GvHD developed, the fourth patient with cutaneous and liver GvHD had been taken off tacrolimus on day 68 due to concerns of graft failure. GvHD resolved with treatment in each case. None of these patients developed chronic GvHD and none have died [median follow-up of 0.66 (range 0.91 - 2.0) years post alloBMT].
Nine patients received checkpoint therapy following alloBMT: anti-PD-1: Pembrolizumab n = 1, Nivolumab n= 6, anti-CTLA4: Ipilimumab n= 3 (one patient received nivolumab and ipilimumab). Eight patients had received nonmyeloablative conditioning; 5 received haploidentical allografts. Six received treatment for relapse of their hematologic malignancy, 1 for relapsed pediatric sarcoma, and 2 for newly diagnosed lung cancer. The median time to initiation of checkpoint inhibitor therapy was 1.2 (range: 0.8 - 5.8) years post alloBMT. Patients received a median of 5 (range 1 - 24) cycles of therapy. There was 1 case of Grade II aGvHD; stage 3 cutaneous GvHD when DLI from a 10/10 matched unrelated donor was given for relapsed disease after ipilimumab. This resulted in GvHD which was not accompanied by the desired graft-vs-leukemia effect. There were no other cases of acute or chronic GvHD in this group. There were 4 tumor-related deaths: pediatric sarcoma (1), lung cancer (1), and AML (2). The median follow-up for this group is 2 years (range 0.85 - 8.0) post alloBMT.
Conclusions: In this small series, the incidence and severity of GvHD seen in patients who received checkpoint inhibitors was similar to that seen in patients treated with PTCy as GvHD prophylaxis without checkpoint inhibitors. GvHD was seen in patients treated with checkpoint inhibitors prior to alloBMT, but was generally mild and readily controlled and there were no associated deaths. In patients treated with checkpoint inhibitors after alloBMT, the only case of GvHD occurred after the patient received DLI. We caution that use of checkpoint inhibitors in closer temporal proximity to transplant might well be associated with increased risk of GvHD or severity of GvHD.
Borrello:WindMIL Therapeutics: Equity Ownership, Patents & Royalties, Research Funding; Celgene: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding. Wagner-Johnston:Seattle Genetics: Research Funding. Smith:Celgene: Consultancy, Other: member of DSMB.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal