Abstract
Introduction: Despite the recent identification of the Ph-like subgroup of B-cell precursor Acute Lymphoblastic Leukemia (BCP-ALL), a large number of BCP-ALL patients lack cytogenetic and molecular defined lesions. To get a higher resolution and a broader molecular view of relapsed BCP-ALL, we designed a multi-omics study to reveal age-overriding relapse-driving alterations that may unravel novel molecular targets.
Methods: We studied 150 paired samples (initial diagnosis: ID; relapse: REL; complete remission: CR) from 50 patients without known translocations. The cohort consisted of 24 adult and 26 pediatric patients with minimal residual disease < 0.05 % at CR. All patients were treated in population based German study trials (GMALL, BFM). We examined the mutational and copy number status via exome sequencing, obtained expression profiles and fusion-genes via RNA-sequencing and the methylation status via Illumina Methylation Array.
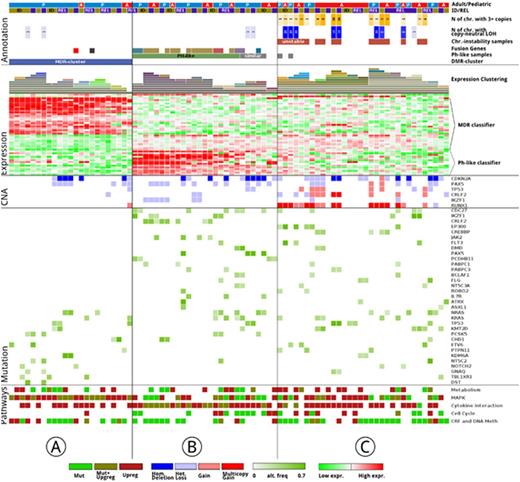
Results: With a lenient approach detecting drivers and passengers, we identified significantly more mutations in REL compared to ID samples (adult median: 52 vs 38; pediatric median: 39 vs 27). In addition, we detected 4 hypermutators (more than 100 mutations per sample), 2 were pediatric and 2 were adult samples, 3 of which were REL samples. The most recurrently mutated genes were KRAS (n=15), NRAS (n=15), TP53 (n=13), CDC27 (n=13), KMT2D (n=11), IKZF1 (n=11), CREBBP (n=10) and FLT3 (n=6; Figure 1), with mutations present in both age cohorts. NT5C2, SYK and CHD1 were exclusively mutated in the pediatric cohort with at least 3 mutations. NT5C2 was also specific for early REL. Of all REL mutations, 225 mutations (14%, mean: 4 mutations/patient) were sub-clonal (under < 5% mutation frequency) at ID.
Copy number alterations (CNA) varied greatly among pediatric and adult samples: 6% of pediatric and 18% of adult samples had aneuploidies and or copy neutral loss of heterozygosity of whole chromosomes. Chromosomal aberrations at ID persisted at relapse (100 %). Particular targets of CNA affected well-described genes like CDKN2A, CDKN2B, PAX5 on chr9p. Genes preferentially subjected to homozygous deletions were VPREB1 (n=6), SH2B3 (n=4), and ETV6 (n=2). All SH3B2 deletions were found in pediatric samples.
On the epi-genomic level, the principal component analysis of the most variable CG-sites revealed a stable methylation profile during the course of the disease. However, we found a clear separation into a smaller pediatric-dominated cluster (n=24; 20 pediatric, 4 adult) and a larger mixed-age cluster (n=76; Fig. 1, Cluster A). Differentially methylated regions, affecting a total of 269 genes, characterized the separation of the smaller cluster, henceforth called Methylation Deregulated (MDR) cluster.
The samples of the MDR cluster showed also a distinct gene expression profile by RNA-seq supporting a tight connection between the methylation status and its transcriptional program. A subset of 97 genes was differentially expressed including MAPK and PDGFR genes as most prominently deregulated. Additionally we defined a MDR expression classifier comprising 30 genes (Fig. 1). On the mutational level, the MDR samples had 20 % fewer mutations (mean: 25.3) compared to the remaining samples (mean: 31.3) and fewer CNVs for the most frequently affected genes.
Characterising the non-MDR samples, a third of those were categorized as Ph-like ALL using the 15 gene classifier in an unsupervised clustering; this signature also coincided with the presence of well-known fusion-genes (Fig. 1, Cluster B). The remaining samples were defined by chromosomal instability (CI; Fig. 1, Cluster C). In the CI cluster, mutations in epigenetic regulators were twice as frequent when compared to the remaining samples.
Conclusions: We describe three distinct clusters in relapsed BCP-ALL, which are characterized by a different genetic alterations: a novel MDR cluster by distinct methylation changes, the Ph-like cluster by gene fusions and the CI cluster by chromosomal instability. The cluster assignment was stable over the course of the disease. All clusters occurred in pediatric and adult patients, with the methylation-driven cluster predominantly in pediatrics. The MDR cluster showed significantly fewer mutations and CNVs compared to the other two clusters. The MDR samples showed activation of the MAPK signaling pathway pointing to actionable therapeutic targets.
Gökbuget:Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal