Abstract
Introduction: Ibrutinib is approved for treatment of patients (pts) with treatment-naïve or relapsed CLL. We previously reported poor outcomes for pts discontinuing ibrutinib; however, long-term outcomes were not reported.
Methods: We present the outcomes for 90 pts who discontinued ibrutinib from a total of 320 pts treated with ibrutinib-based regimens on clinical studies from 2010-15 at M.D. Anderson Cancer Center.
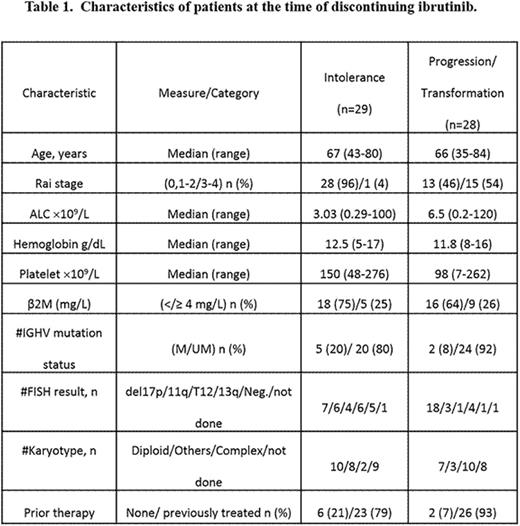
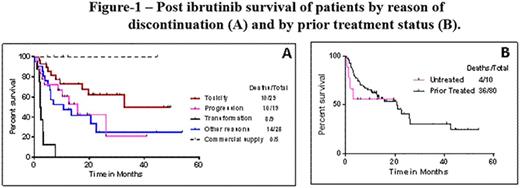
Results: Ninety (28%) pts discontinued ibrutinib. Forty pts (44%) were alive at the time of last follow up; 50 (56%) have died. Among the 90 pts, 47 received ibrutinib monotherapy, 31 ibrutinib plus rituximab and 12 ibrutinib with bendamustine and rituximab; 80 pts were relapsed/refractory (r/r) and 10 were treatment-naive. The median time to discontinuation for the 90 pts was 15 months (range 1.2-54), 19 months (5-47) for the untreated and 14.5 months (1.2-54) for r/r pts. Overall, causes and median time for discontinuation were: disease transformation [n=9, 10%; 13.2 mo.]; progressive CLL [n=19, 21%; 22.3 mo], intolerance/toxicity [n=29, 32%;16 mo]; transition to commercial supply (n=5;26 mo.); miscellaneous [n=28, 31%; 10.4 mo] (9 stem cell transplantation, 8 other cancers, 2 sudden death, 6 sepsis and previous comorbidities, 3 unknown). Reasons for discontinuation after first-line ibrutinib were - disease transformation [n=2]; intolerance [n=6; 3 atrial fibrillation and arrhythmias, 2 bleeding and 1 pneumonia]; transition to commercial supply (n=1) and death due to unknown cause (n=1). In r/r pts, reasons for discontinuation were: disease transformation [n=7]; intolerance [n=24; 3 atrial fibrillation, 2 bleeding and 1 pneumonia]; progression [n=19], 27 miscellaneous reasons and 4 for commercial supply. Characteristics of pts who discontinued due to intolerance and progression/transformation are summarized in Table-1. Median time to discontinuation was 16 months (1.2-49 months) for intolerance and 19 months (2-54 months) for progression/transformation. Survival of pts according to the cause of discontinuation is shown in Figure-1A. Median survival of pts was: 33 months for intolerance,16 months in progressive disease and 2 months in disease transformation. Survival of pts according to prior treatment status is shown in Figure-1B. We then assessed response to subsequent therapies among the 19 pts who developed progressive CLL on ibrutinib. Eight of 19 pts (42%) responded to subsequent therapy:5/8 (62%) achieved PR on venetoclax-based therapies (3/5 also failed idelalisib prior to commencing venetoclax); 2/8 (25%), responded to ofatumumab monotherapy;1/10 patients treated with idelalisib or duvelisib-based therapy responded.
Conclusions: Ibrutinib discontinuation is common. Subsequent survival correlates with reason for discontinuation; disease transformation has very poor outcome, while patients who develop progressive CLL have a median survival of 16 months. Development of effective salvage strategies for patients with progression/ transformation on ibrutinib therapy is of critical importance. Ideally, these strategies should be guided by the knowledge of the molecular mechanisms of resistance in each patient. Of note, response rates to venetoclax-based therapy were relatively favorable. Despite having superior outcomes to those patients who progress, those who develop treatment-limiting toxicity have a median survival of 33 months and development of guidelines for managing toxicity to allow continued therapy is of importance, as is developing successful salvage options for those unable to continue.
# at baseline
Thompson:Pharmacyclics: Consultancy, Honoraria. Jain:Incyte: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Servier: Consultancy, Honoraria; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Novimmune: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Genentech: Research Funding; Seattle Genetics: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Infinity: Research Funding. Burger:Roche: Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Gilead: Research Funding; Portola: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Research Funding. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Wierda:Novartis: Research Funding; Acerta: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal