Abstract
Introduction. The combination of Lenalidomide (Len) and rituximab has been investigated in patients (pts) with treatment-naive (TN) and relapsed (R) CLL with an overall response rate (ORR) ranging between 66% and 85%. Fludarabine refractoriness and a median daily Len dose < 5 mg were the only factors predictive of a worse response to therapy in these reports. We, therefore, conducted a phase II study to evaluate how clinical and biological prognostic factors correlate with response.
Methods. Pts were eligible for this study if they had treatment indications per IWCLL 2008 criteria, WHO PS ≤2 and adequate hepatic and renal function. Len was started on day 9 of cycle 1 at 10 mg orally and administered daily indefinitely. Rituximab, 375 mg/m2, was administered every 28 days for 12 cycles. Response was assessed at month 3, 6 and every 6 months thereafter. Pts in this study returned to our center at time of response assessment and were allowed to receive R infusion at their oncologist's office. The primary end point of this study was ORR.
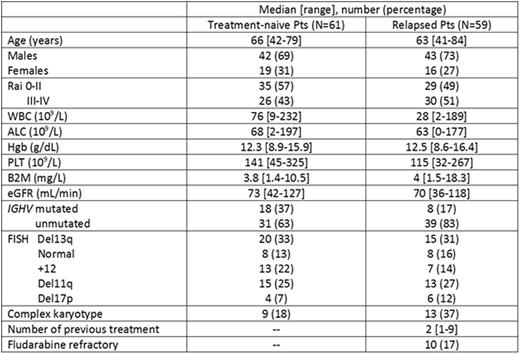
Results. One-hundred-twenty pts (61 TN and 59 R) were enrolled between 1/2012 and 11/2014; 83 (69%) pts received their R infusion locally. Baseline pts characteristics are shown in the Table.
The median number of cycles administered was 16 [1-54] in TN and 20 [1-59] in R pts. Fifty-five TN and 53 R pts were evaluable for response. Twelve pts discontinued therapy before the first response assessment [toxicity (8), loss to follow-up (3), and death (1)]. ORR was 73% in TN and 64% in R pts, CR/CRi rate was 35% in TN and 28% in R pts. MRD eradication was achieved in 16% of TN and 2% of R pts. Median time to response was 11 [8-14] and 6 [5-7] months in TN and R pts. Median response duration (RD) has not been reached for TN pts and was 37 (29-45) months for R pts; 28% of TN and 62% of R pts have progressed. Characteristics predictive of higher ORR were: B2M ≤ 4 mg/L in TN pts (85% vs 55%, p=0.03); age < 65 (p=0.05), Rai 0-II (p=0.03), B2M < 4 (p=0.009) and eGFR > 60 mL/min in R pts; on MVA, only B2M maintained its association (84% vs 46%; OR 5, 95% CI 1.1-10; p=0.03). FISH and IGHV status did not predict ORR. Characteristics associated with longer RD were age ≤ 65 years (p=0.009) and non-complex karyotype (p=0.05) in TN pts; on MVA, only age maintained its association (not reached vs 33 months; HR 5; 95% CI 1-25.6; p=0.05); < 2 prior treatments was the only factor associated with RD in R pts (48 vs 20 months, p=0.03).
Treatment-associated G 3-4 toxicities occurred in 28% of TN and 30% of R pts. Most common toxicities were: infections (10% of TN and 22% of R pts), thromboembolic complications (6%, both groups), skin rash (6% in TN pts only), and tumor flare reaction 6% (TN pts only).
Forty-three (70%) TN and 50 (85%) R pts have discontinued treatment, median time to failure (TTF) was 22 [16-28] and 34 [25-43] months, respectively. Reasons for interruption were: progression in 13 (30%) TN and 25 (50%) R pts, toxicity in 25 (57%) TN and 20 (40%) R pts, pt/physician choice in 3 (7%) TN and 4 (8%) R pts, and other cancers in 2 (5%) TN and 1(2%) R pt. Factors predictive of a longer median TTF were eGFR ≥ 60 mL/min in TN pts (23 months vs 7 months, p=0.05) and early Rai stage in R pts (41 vs 36 months, p=0.04).
At a median follow-up of 34 [1-53+] months, 4 (7%) TN and 14 (24%) R pts died, with a 4-year OS of 90% and 70%, respectively. Factors associated with shorter median OS were advanced Rai stage in TN pts (75% vs 100%, p=0.02), and > 2 prior treatments in R pts (on MVA; 50% vs 90% 4-years OS; HR 5.2; 95% CI 1.3-21; p=0.02). When comparing pts treated at our center to those receiving R infusion in the community, no significant differences in ORR, G3-4 toxicity rate or OS were observed.
Conclusions. In our experience, the combination of Len and R is able to induce responses in 73% of TN and in 64% of R pts. Pts age <65 years, with a history of <2 prior treatments and with B2M <4 were more likely to respond and to have a longer response duration. No differences were seen based on pts' FISH abnormalities and IGHV status. Similar results in terms of efficacy and toxicity were observed in pts treated at our center or managed in close cooperation with local providers. With the expanding number of active treatment options for pts with CLL, the identification of clinical and biological factors predictive of response is becoming increasingly important.
Thompson:Pharmacyclics: Consultancy, Honoraria. Jain:Pfizer: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Abbvie: Research Funding; Novartis: Consultancy, Honoraria; Infinity: Research Funding; Genentech: Research Funding; Servier: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Incyte: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding. Burger:Pharmacyclics: Research Funding. Wierda:Gilead: Research Funding; Abbvie: Research Funding; Acerta: Research Funding; Novartis: Research Funding; Genentech: Research Funding. Kantarjian:Bristol-Myers Squibb: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal