Abstract
Introduction
While lenalidomide (LEN) is the standard of care for treatment of red blood cell (RBC) transfusion-dependent (TD) lower-risk myelodysplastic syndromes (LR-MDS) with chromosome 5q deletion (del 5q), it is widely used off-label in the non-del5q setting. In the MDS-002 and MDS-005 studies, 26% of TD non-del5q LR-MDS patients became RBC transfusion independent after LEN treatment. National Comprehensive Cancer Network (NCCN) clinical guidelines list LEN as a second line treatment option for TD anemia in lower-risk non-del 5q MDS after hypomethylating agents (HMAs). Thatrecommendationhas led to wide use of HMAs as frontline therapy after erythroid stimulating agents (ESA) failure in LR-MDS. The response rate to LEN after HMA failure, however, is not known, as MDS-002 and MDS-005 excluded patients previously treated with HMAs. To assess the best order of LEN and HMA in optimizing response potential in lower-risk MDS, we examined response rates to each drug when treatment order (LEN followed by HMA or HMA followed by LEN) differed.
Methods
We identified patients with LR- MDS (International Prognostic Scoring System (IPSS) low or intermediate-1 (int-1) risk groups) within the MDS Clinical Research Consortium database who received both LEN and HMA as first or second line therapy after ESA failure or patients who had low chance of response to ESA. We excluded patients with isolated del5q or del q + one additional cytogenetic abnormality. The primary objective was to compare rates of erythroid hematological improvement (HI-E), defined using 2006 International Working Group criteria (IWG 2006), between patients who received LEN as first line therapy followed by HMA as second line (LEN 1st line group) versus those who received LEN as second line therapy after HMA (LEN 2nd line group).
Results
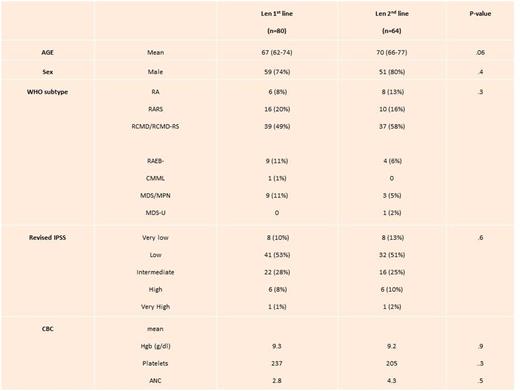
We identified 144 patients who received both HMA and LEN as first and second line therapies: 80 patients were in group 1 (LEN 1st line) and 64 patients were in group 2 (LEN 2nd line). Baseline characteristics between the two groups are summarized in Table 1. There were no statistically significant differences between the 2 groups.
The rate of HI-E was 20% (16/80) for the LEN 1st line group compared to 11% (7/64) in the LEN 2nd line group. (p=.046). There were no differences in response rates to HMA between the two groups: In the LEN 1st line group, response to 2nd line HMA was 30% (24/78) compared to 39% (25/64) for those who received HMA as first line (p=.2).
There was no difference in overall survival (OS) between the two groups, The median OS was 79 months (mo) the LEN 1st line group compared to 61 mo in Len 2nd line group (p= .4). The rate of AML transformation was 9% in Len 1st line group compared to 22% in Len 2nd line group rate (p=.03). There was no difference in AML free survival (78mo versus 61mo respectively, p=0.4). In multivariable analyses adjusting for age, sex, and IPSS-R order of treatment still did not impact overall survival.
Conclusion
LEN yields a higher rate of HI-E in LR- MDS when used as first line therapy, but responses to HMAs were similar when used before or after LEN. The rate of AML transformation was lower when LEN was used as first line. The order of treatment does not impact overall survival. Lenalidomide should be used prior to HMA if it is to be considered for treatment of anemia in non-del5q LR-MDS.
Komrokji:Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy; Boehringer-Ingelheim: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Roboz:Agios, Amgen, Amphivena, Astex, AstraZeneca, Boehringer Ingelheim, Celator, Celgene, Genoptix, Janssen, Juno, MEI Pharma, MedImmune, Novartis, Onconova, Pfizer, Roche/Genentech, Sunesis, Teva: Consultancy; Cellectis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal