Abstract
Myelodysplastic syndromes (MDS) are clinically heterogeneous hematological malignancies with a propensity to evolve to acute myeloid leukemia. Recent studies have suggested that the different MDS risk subgroups are characterized by distinct hematopoietic architectures [Will 2012; Pang 2013], but the contribution of the specific hematopoietic stem cell (HSC) compartments to the aberrant differentiation responsible for the MDS phenotype and their role in disease progression are not fully understood. Although it is known that the cells that maintain the disease in the low-risk (LR) subgroup reside in the primitive long-term (LT)-HSC subset [Pang 2013], little is known about the hierarchical organization of high-risk (HR) disease. A detailed understanding of the architecture and dynamics of the HSC populations during MDS evolution could precisely identify the HSC responsible for relapse, and molecular characterization of these cells may allow for effective disease monitoring and progression prevention.
With this purpose, we have immunophenotypically characterized the stem and progenitor cell compartments in 75 BM samples isolated from 67 patients with low- and high-risk MDS at different stages of the disease and have analyzed the variant allelic frequency in the HSC compartment of 280 leukemia-relevant genes by targeted next-generation sequencing.
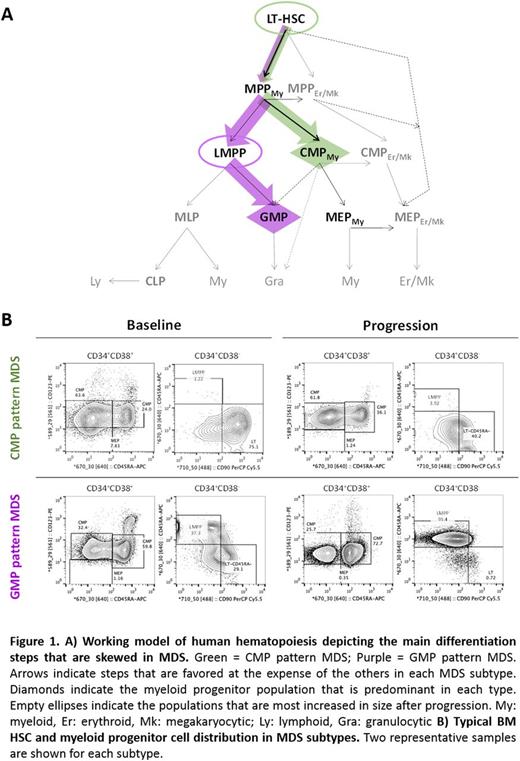
Our analyses show that MDS can be classified into 2 biological entities based on the cellular architecture of the stem and progenitor compartments and independently of the risk stratification. Indeed, when compared to 8 healthy donor controls, 10 patients at baseline (38%; 3 LR, 7 HR) showed a "CMP pattern of differentiation" characterized by a decreased number of granulocytic-monocytic progenitors (GMP) (5.3-fold; p=0.002), with no significant changes in the number of the common myeloid progenitors (CMP) and megakaryocytic-erythroid progenitors (MEP). Decreased GMP counts resulted in a higher frequency of the CMP (2.7-fold; p<10-4), which, therefore, was the predominant population within the progenitor compartment. A deeper immunophenotypic analysis using the novel hematopoietic hierarchy defined by Dick et al. [Notta 2016] showed loss of functional heterogeneity of the CMP (p<10-4) and MEP (p<0.05) populations, as well as of the multipotent progenitors (MPP) (p<10-4). Although it was not expanded, this population was skewed towards the myeloid lineage, therefore suggesting aberrant differentiation of the upstream LT-HSC. Consistently, the LT-HSC compartment was significantly increased during blast progression compared to baseline patients (9.9-fold; p<10-4) and patients who developed ineffective hematopoiesis after therapy (19.4-fold; p=0.002).
In contrast, a second subgroup of baseline patients (62%; n=16; 10 LR, 6 HR) showed a "GMP pattern of differentiation" characterized by increased frequency of the GMP population (1.71-fold; p<10-4), a sharp decrease in the number of the MEP (4.4-fold; p=0.003) and unchanged CMP counts. Although the analysis of the MPP population in these patients showed loss of heterogeneity, indicating aberrant differentiation of the LT-HSC population, "GMP pattern" patients had a slightly reduced number of LT-HSC (2.7-fold; p=0.06) and increased LMPP (4.4-fold; p=0.017), which explains why the GMP were the most abundant myeloid progenitors. Consistently, the LMPP population was significantly increased in "GMP pattern" patients who failed therapy compared to baseline (15.2-fold; p<0.001), with no significant differences between leukemic progression and ineffective hematopoiesis. A milder but significant increase in the number of MPP (5.5-fold; p=0.001) suggests that both multipotent progenitor subsets contribute to treatment failure.
Importantly, these two distinct cellular architectures were maintained during the course of therapy in 42 MDS patients treated with hypomethylating agents (HMA), which confirms previous studies showing that HMA cannot overcome aberrant differentiation in MDS [Will 2012; Craddock 2013; Woll 2014].
Collectively, our study identifies two biologically distinct entities of MDS, in which different HSC populations maintain skewed differentiation and separately contribute to disease progression. Mutational analysis of the HSC compartment at different disease stages supporting our biological classification of MDS will be further discussed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal