Abstract
Myelofibrosis (MF) is caused by genetic abnormalities involving the thrombopoietin (TPO) axis (mutations in either the TPO receptor, MPL, MPL-signaling, JAK2, or MPL partners, Calreticulin). Furthermore MF patients have elevated serum TPO levels. By contrast with other MPNs, MF is also associated with reduced GATA1 content in MKs (Vannucchi et al, Am J Pathol 2005;167:849). In 2014, we suggested that the "element" that specifically links TPO signaling with de-regulated Gata1 expression in MKs represents the phenotypic modifier leading to MF development and presented data suggesting that this "element" is a ribosomal deficiency, which hampers GATA1 mRNA translation (Gilles et al, Blood 2014; 124:4585 abstract).
Support for GATA1 deficiency in MKs as a "phenotypic modifier" in MF was provided by the observation that mice carrying a hypomorphic mutation (Gata1low) which develop MF have reduced transcription of Gata1 in MKs (Vannucchi et al, Blood 2002;100:1123). These mice are subjected to continuous "erythroid stress" due to reduced RBC half-life and contain increased numbers of bipotent Ery/MK precursors with a unique phenotype (Vannucchi et al, Blood 2000; 95:2559) similar to that of the bipotent Ery/Mk precursors induced in vitro from cord blood by a chemical MPL stimulator (Belay et al, Blood 2015; 125:1025). These considerations led us to investigate the activation state of the TPO/Mpl axis in this model.
In Gata1low and wild-type mice, TPO mRNA was expressed by bone marrow (BM), spleen and liver. The greatest levels (by 300-fold) were detected in the liver. Gata1low livers expressed TPO mRNA at 6-fold greater levels than wild-type livers. TPO protein was detected in BM, spleen, liver, peritoneum and plasma. Gata1low plasma contained TPO levels 2-times greater than plasma from mice with similar levels of thrombocytosis (778±101 vs 451±51pg/mL in bleed wild-type mice and 351±31pg/mL in Mplnull mice) suggesting that the TPO axis is activated in Gata1low mice.
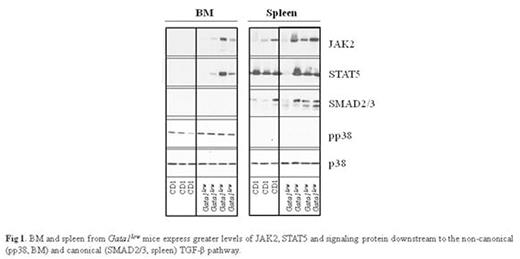
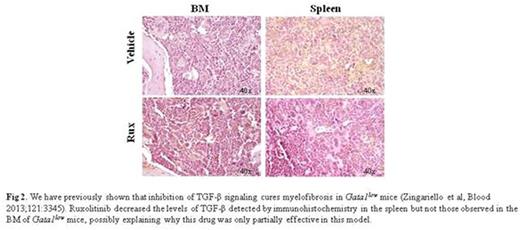
This hypothesis was further tested by determining that Gata1low LSK expressed levels of Mpl mRNA 3-times greater than wild-type cells, but expressed on their surface levels of MPL 2-times lower than wild-type LSK. This reduction was similar to the level of MPL in LSK from wild-type mice treated with TPO, suggesting that TPO stimulation down-modulated MPL. The MPL signaling partners JAK2 and STAT5 were barely detectable in wild-type BM but were detected at robust levels in Gata1low BM and spleen (Fig 1) suggesting TPO stimulated greater JAK/STAT signaling. The role of the TPO/Mpl axis in Gata1low mice was further investigated by determining the effects of the JAK/STAT inhibitor ruxolitinib (90mg/Kg/Day or vehicle for 2 weeks, three females per group). This treatment induced a slight anemia (38±1 vs 41±4), reduced spleen size (240±60 vs 380±80 mg in vehicle and 350±80 in untreated Gata1low mice), did not increase BM cellularity (10±1.3x106 vs 9.6±1.0x106 in vehicle and 18±2x106 in wild type cells/femur), reduced BM fibrosis (2.7±0.3 vs 7.9±0.1 arbitrary units of Gomori staining) and reduced the abnormal TGF-β content present in the spleen but not that of BM (Fig 2).
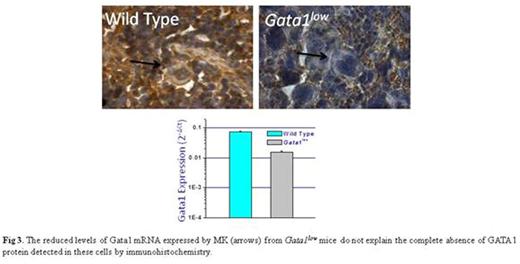
The discrepancy between the modestly reduced Gata1 mRNA levels and the undetectable GATA1 protein observed in Gata1low MKs (Fig 3) led us to investigate the expression of ribosomal related genes in these mice. Microarray analyses of BM and spleen from Gata1low and wild-type mice indeed identified a discordant ribosome signature solely in BM. This signature included reduced expression of RPS24 (-2-fold, p=0.04) and RPS26 (-3-fold, p=0.03), two genes mutated in a subset of patients with Diamond Blackfan Anemia associated with ribosomapathies leading to reduced GATA1 mRNA translation (Danolova et al, Disease Model&Mechanism 2015;8:1013) and SBDS (-2-fold, p=0.01), another hypomorphic gene in the inherited BM failure state Schwachman-Diamond syndrome which regulates ribosome biogenesis (Zambetti et al, Haematologica 2015;100:1285). In addition, electron microscopy revealed that Gata1low MKs contain poorly developed endoplasmic reticulum with rare polysomes.
We conclude that Gata1low mice that develop MF have an activated TPO/MPL axis and an abnormal ribosomal signature which may lead to reduced Gata1 translation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal