Abstract
Background: Patients (pts) with polycythemia vera (PV) and essential thrombocythemia (ET) suffer from disease related events linked to risk of vascular events, splenomegaly, progression to myelofibrosis/acute leukemia, and disease related symptoms arising from both elevated cytokines and issues of vascular flow. Impact of front line therapy on ET/PV symptoms has not been reported in a systematic fashion.
Methods: MPD-RC 112 trial (NCT01258856) enrolled cytoreductive therapy naïve (hydroxyurea [HU] <3 mo) pts with high risk ET or PV within 3 years of diagnosis. Pts were randomized (1:1, stratified by ET vs PV) to either response adjusted pegylated interferon alpha 2a (PEG) or HU. MPN symptoms, treatment toxicities, and quality of life (QoL) were measured by the MPN-SAF and EORTC QLQ-C30 at baseline and 3, 6, 9, and 12 mo. PEG related toxicities were assessed with 5 questions on PEG arm only. European Leukemia Net (ELN) complete hematological responses (CHR) at 12 mo were determined by central blinded review. Individual time point and longitudinal group comparisons were based on mixed models adjusting for age. T-tests were used to compare change scores at 12 mo between pts with and without CHR.
Results:
Patients:
MPD-RC 112 enrolled 168 pts from 9/2011 to 7/2016, with 75 pts included in the interim analysis. 73 (97%; HU 37, 95%; PEG 36, 100%) completed surveys at baseline of which 66 (90%; HU 30, 81%; PEG 36, 100%) completed at least 1 survey during treatment. Median age was 60 (range 19-84) with 32 (44%) females; 30 (41%) / 43 (59%) with ET / PV; 19 (26%) had a history of thrombosis; and 16 (22%) had palpable spleen. Baseline characteristics where balanced between arms with the exception of age (median age: HU 66, PEG 54; p<0.001).
Baseline Symptom Burden/QoL:
Mean MPN-SAF Total Symptom Score (TSS, scale 0 [absent]-100 [worst imaginable]) was 15.4 (SD 12.5; range 0-52.2) with means of 12.6 (SD 11.7) / 17.3 (SD 12.7) for ET / PV which were somewhat better than reported means of a previous cohort receiving any line of treatment (ET mean 18.7, SD 15.3; PV mean 21.8, SD 16.3; Emanuel RM, JCO 2012). Symptoms (scale 0 [absent]-10 [worst imaginable]) with the highest prevalence (score >0) included fatigue (65/73, 89%) and insomnia (47/73, 64%). The symptom with the lowest prevalence was fever (8/72, 11%). Mean QLQ-C30 global health status/QoL (GHS/QoL) was 73.3 (SD 19.1) which is comparable to a general healthy population (mean 71.2, SD 22.4) and better than a general cancer population (mean 61.3, SD 24.2; QLQ-C30 Reference Manual 2008). TSS, symptoms, and QoL did not differ between arms.
Impact of Therapy on Symptom Burden/QoL:
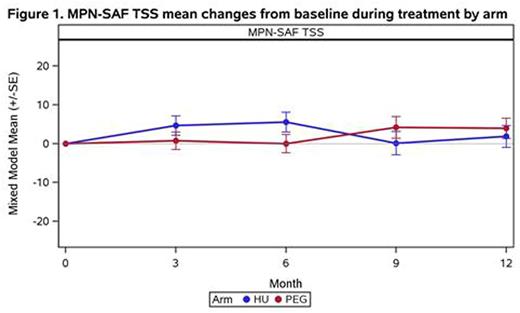
On HU, pts initially experienced worsening of TSS, fatigue, early satiety, itching, bone pain, and fever (all p<0.05) with some symptoms returning to baseline after 6 mo (Table 1). On PEG, pts experienced improved abdominal pain and discomfort, but worsening of headache, cough, injection site irritation, blurry vision, and vision change (all p<0.05). Sad mood did not worsen on PEG, although this may be confounded by concurrent use of mood stabilizers. Concentration problems on PEG were significantly worse at 9 mo (p=0.04). Both arms experienced worsened QoL (MPN-SAF single item: HU p=0.009, PEG p=0.003). In comparing between arms, TSS significantly differed (p=0.009) with higher symptoms on HU vs PEG at 3 and 6 mo, but lower symptoms at 9 and 12 mo (Figure 1). Similar descriptive profiles were observed for most individual symptoms; however, the only symptom profiles which significantly differed between arms was early satiety (p=0.03).
ELN Response and Symptom Burden/QoL:
Among 62 pts with symptom and response data at 12 mo, CHR rate was 37% (23/62). Change in sad mood and sexuality were the only significant differences between pts with and without CHR regardless of arm though in the unanticipated direction (sad mood: mean change 1.5 vs -0.8, p<0.001; sexuality: mean change 2.33 vs 0.3, p=0.03).
Conclusions: PEG was associated with an initial decrease in MPN symptom burden when compared to HU. However, with longer duration of therapy, this superiority dissipated. Patient-reported PEG toxicities worsened over time. On both arms, symptom worsening was observed in pts achieving an ELN CHR, possibly leading to the observed negative impact on mood and sexuality. Future research on the impact of PEG on disease progression and molecular features will be important to assess whether increased side effects with PEG are justified.
Mesa:CTI: Research Funding; Celgene: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Incyte: Research Funding; Galena: Consultancy; Ariad: Consultancy; Novartis: Consultancy. Harrison:Incyte Corporation: Honoraria, Speakers Bureau; Baxaltra: Consultancy, Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Shire: Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses, Research Funding, Speakers Bureau. McMullin:Novartis: Honoraria, Speakers Bureau. Yacoub:Alexion: Honoraria; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau. Kiladjian:Novartis: Honoraria, Research Funding; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mead:Novartis: Honoraria, Research Funding, Speakers Bureau. Kessler:Pfizer: Consultancy; Octapharma: Consultancy, Research Funding; Novo Nordisk: Consultancy, Research Funding; Grifols: Consultancy; Genentech: Consultancy, Research Funding; Biogen: Consultancy; Bayer: Consultancy, Research Funding; Baxalta: Consultancy, Research Funding; LFB: Other: Member of DSMB. Ritchie:Pfizer: Honoraria; Novartis: Honoraria; Arian: Speakers Bureau; Celgene: Speakers Bureau; Incyte: Speakers Bureau. Schlenk:Pfizer: Honoraria, Research Funding; Amgen: Research Funding. Mascarenhas:Promedior: Research Funding; Janssen: Research Funding; CTI Biopharma: Research Funding; Novartis: Other: DSMB , Research Funding; Roche: Research Funding; Incyte: Other: Clinical Trial Steereing Committee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal