Abstract
Background: Post-transplant lymphoproliferative disorder (PTLD) is an uncommon, heterogeneous disease that occurs in the setting of immune suppression following solid organ transplantation. As the transplant population ages over time, the spectrum of PTLD histologies and their treatment has evolved; we report here our recent experience with PTLD from a large multi-organ transplant program.
Methods: We identified patients from the Multi-Organ Transplant Program at University Health Network (UHN) who were diagnosed with PTLD between January 1, 2000 and December 31, 2015. We describe the characteristics and outcomes of this cohort, with a focus on the outcome of patients with the diffuse large B cell (DLBCL) subtype of PTLD treated in the rituximab era.
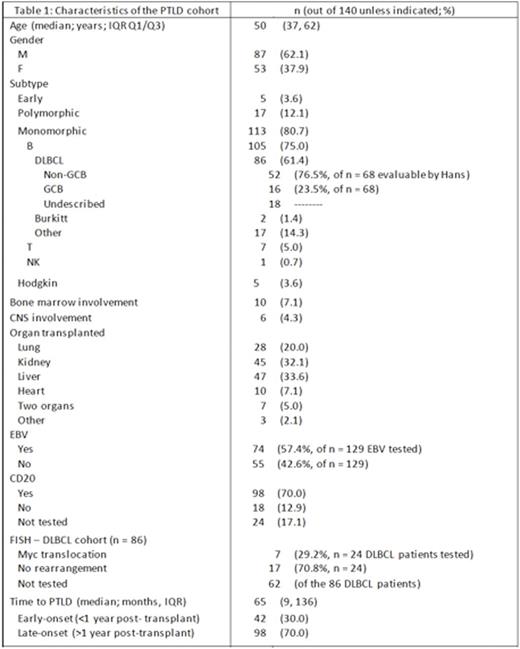
Results: A total of 140 patients were diagnosed with PTLD at UHN during this time period (Table 1): 38% were female and median age at time of diagnosis was 50 (interquartile range, IQR Q1/Q3, 37 to 62 years). The most commonly implicated transplants were liver and kidney (33% and 32%), as well as lung (20%) and heart (7%). The median time from organ transplantation to PTLD diagnosis was 61 months (IQR 9 to 136), with 70% of cases occurring more than 12 months following solid organ transplant. Pathologically, the majority of the patients had monomorphic PTLD, with DLBCL (n = 86) being most common. Where classifiable by the Hans algorithm (n = 68), the majority of the DLBCL types were of the non-germinal center B cell type (non-GCB, 76.5%). Of 24 DLBCL patients with available FISH, 7 had a MYC translocation (29%).
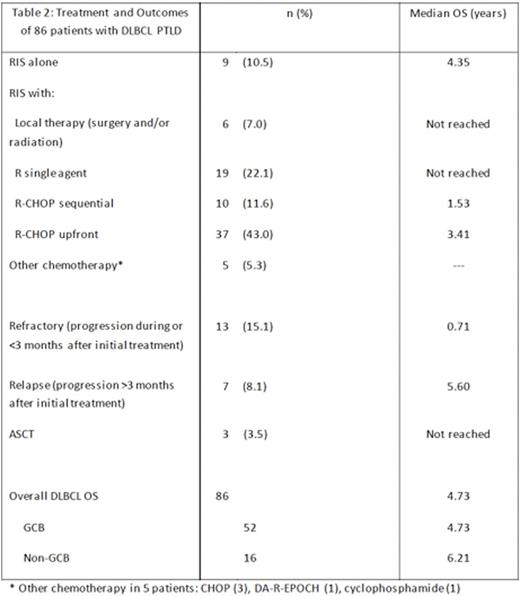
Treatment and outcome varied by PTLD subtype (Table 2). For the overall cohort (n = 140), the median OS was 5.1 years. Highlighting competing risks, less than half of patients died of PTLD, with the remainder of mortality due to complications of solid organ transplantation, or less commonly, due to treatment-related mortality. Polymorphic PTLD (n = 17) was treated in a variety of ways ranging from reduction in immunosuppression (RIS) alone to R-CHOP; median OS was 10.3 years. Less common histologic subtypes (Hodgkin, Burkitt, T cell) were treated with therapies specific to those lymphomas. For DLBCL (n = 86), treatment consisted of RIS alone (10.5%; OS 4.4 years) or RIS followed by local treatment (involved field radiation or surgical resection) (7.0%; OS not reached); single agent rituximab (R) (22.1%; OS not reached); sequential R followed by CHOP (11.6%; OS 1.5 years), or upfront R-CHOP (43.0%; OS 3.4 years). Median OS for patients classified as non-GCB was not worse than those classified as GCB (OS 6.2 vs. 4.7 years, p = 0.93).
In the DLBCL cohort, 20 patients had progression during or after first line therapy, with 14 patients receiving additional treatment. Patients with early relapse during initial therapy or within the first 3 months (n = 13) did poorly compared to patients with later relapse occurring after 3 months (n = 7; median OS 0.7 vs 5.6 years, p < 0.01). This was similar to the entire cohort, where 40 patients relapsed and early relapsed/refractory patients also had poor outcomes compared to late relapsing patients (median OS 0.8 vs. 5.1 years, p <0.01). Six patients in the overall cohort had an autologous stem cell transplant for progressive disease; 4 remain in remission after 5 years (range, 1.3 to 5.8 years follow-up).
Conclusion: In this recent retrospective cohort, PTLD was generally late onset and the median OS of all patients was 5 years. The most common histology was DLBCL (non-GCB subtype), but cell of origin did not influence outcome. Patients with early relapse or refractory disease had poor overall survival. Due to competing risks and comorbidities, many PTLD patients continue to have poor outcomes despite modern treatment strategies.
Jain:Roche Canada: Research Funding. Prica:Janssen: Honoraria. Kukreti:Celgene: Honoraria; Amgen: Honoraria; Lundbeck: Honoraria. Kuruvilla:Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Honoraria; Roche Canada: Consultancy, Honoraria, Research Funding; Merck: Honoraria; Seattle Genetics: Consultancy, Honoraria; Lundbeck: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal