Abstract
Introduction: High-dose cytarabine consolidation chemotherapy for acute myeloid leukemia (AML) induces profound transient myelosuppression. Thrombocytopenia is a limiting factor in chemotherapy administration for maintaining dose intensity and schedule as thrombocytopenic-related hemorrhage may contribute to significant morbidity and mortality. Therapeutic and prophylactic platelet (plt) transfusions remain the treatment of choice but the benefits typically last only 3-7 days, are expensive, time consuming, and may induce alloimmunization and transmit infection. PrE0901 was a phase I multi-center study to evaluate the safety and toxicity of eltrombopag, an orally bioavailable thrombopoietin (TPO) receptor agonist, for plt recovery in AML patients (pts) receiving consolidation therapy.
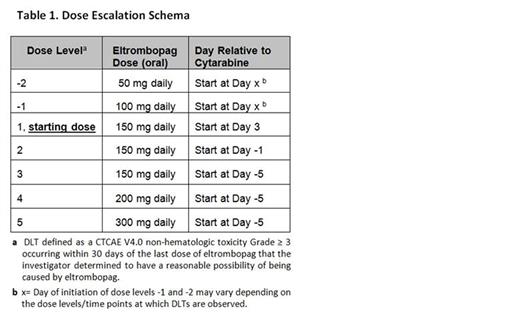
Methods: The primary objectives were to determine the safety, tolerability, and optimal dosing of eltrombopag while describing the kinetics of plt recovery in AML pts receiving intensive consolidation. Plt recovery was defined as a sustained plt count of ≥ 20 x 109/L following the nadir with no plt transfusions in prior 7 days or a continued upward trend in plt count for at least 2 successive measurements despite no plt transfusions in 48 hours. We used a standard 3+3 design utilizing a novel dose escalation/de-escalation strategy for pts in either first or second complete remission, ≥18 and ≤70 years of age, who had an ECOG performance status of 0-2. Cytarabine was administered at 3 g/m2 (1.5 g/m2 for pts >60 years) IV over 3 hours twice daily on days 1,3,5. Eltrombopag at assigned dose was administered with a first cycle of cytarabine only. Planned eltrombopag dose levels -2, -1, 1, 2, 3, 4, and 5 were 50, 100, 150, 150, 150, 200, and 300 mg, respectively (Table 1). Eltrombopag was to start on days X, X, 3, -1, -5, -5, and -5 relative to day of cytarabine start and levels -2, -1, 1, 2, 3, 4, and 5, respectively, with day X being variable depending on timing of observed dose limiting toxicities (DLTs). The eltrombopag was continued until plt recovery or for up to 35 consecutive days, whichever occurred first. An exploratory objective was to determine if eltrombopag had an effect on TPO and EPO blood concentrations in this setting.
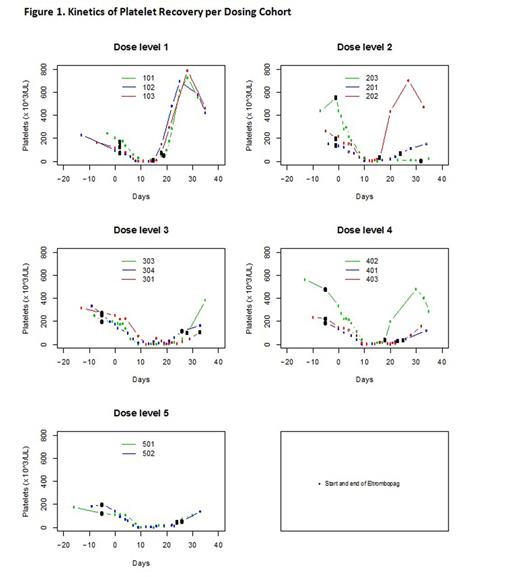
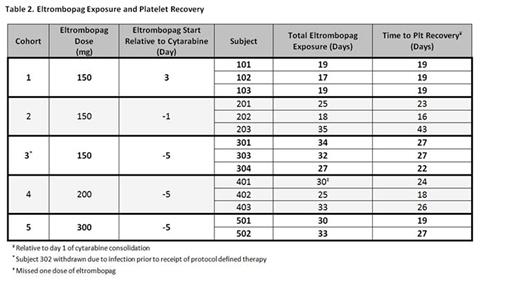
Results: Accrual began on July 31, 2012 and the trial closed on January 8, 2016 upon recommendation after an independent Data and Monitoring Committee reviewed a separate trial of eltrombopag therapy in MDS which demonstrated futility. 104 pts were screened. 54 declined participation and 35 were deemed medically ineligible. A total of 15 eligible pts were enrolled; 14 were treated on study with one pt being withdrawn prior to starting treatment due to infection. Three pts were treated at each of dose levels 1-4 and two were treated on dose level 5. No DLTs were observed. Median time to plt recovery of all pts treated on study was 22.5 (range 16-43) days. Pts in cohort 1 dosed with eltrombopag 150 mg daily starting on day 3 of consolidation demonstrated the fastest plt recovery (median =19 days; range 19, 19, 19) compared to cohort 2 who received 150 mg daily starting on day -1 of consolidation and recovered the slowest (median 23 days; range 16, 23, 43). Dose escalation in terms of starting eltrombopag further in advance of chemotherapy exposure as within cohort 3 (150 mg starting day -5) was associated with a median plt recovery of 27 (range 22, 27, 27) days. Eltrombopag dose intensification with cohort 4 at 200 mg daily and cohort 5 at 300 mg daily was associated with median plt recoveries of 24 (range 18, 24, 26) and 23 (range 19, 27) days, respectively. Kinetics of plt recovery for all cohorts are represented in Figure 1 and duration of eltrombopag exposure for each individual patient is represented in Table 2.
Conclusions: Endogenous cytokine (TPO and EPO) levels varied inversely with plt and hematocrit values and did not appear to be affected by eltrombopag dose / schedule (details to be provided at ASH Annual Mtg). Unusually high screen fail numbers warrant examination to guide future trial design in the post-remission setting. The addition of eltrombopag to high-dose cytarabine consolidation therapy was well-tolerated and no DLTs were observed. The results for cohort 1 [eltrombopag 150 mg daily starting on day 3 of consolidation] demonstrated the fastest plt recovery. Further investigation is needed to define the optimal role, dose, and schedule of eltrombopag in the treatment of chemotherapy associated myelosuppression.
Strickland:Alexion Pharmaceuticals: Consultancy; Ambit: Consultancy; Baxalta: Consultancy; Boehringer Ingelheim: Consultancy, Research Funding; CTI Biopharma: Consultancy; Daiichi Sankyo: Consultancy; Sunesis Pharmaceuticals: Consultancy, Research Funding; Abbvie: Research Funding; Astellas Pharma: Research Funding; Celator: Research Funding; Cyclacel: Research Funding; GlaxoSmithKline: Research Funding; Karyopharm Therapeutica: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal