Abstract
Introduction
Chemotherapy-inducedmyelosuppressionis a major complication for cancer patients causing high rates of morbidity and mortality. Furthermore,myelosuppressionis frequently managed by delaying and/or reducing the scheduled dose, and as a consequence the efficacy of the treatment can be compromised.Since chemotherapy remains the cornerstone for treating many cancers, the development of compounds that inhibitmyelosuppressionwould provide considerable health benefits. By repurposing quizartinib we have identified a novel approach to managemyelosuppression, which led to the development of an effective treatment for a mouse model of acute myeloidleukemia(AML).
Methods
Quizartinib was administered to C57BL/6 mice by oral gavage with a single priming dose of 10 or 30 mg/kg. Fluorouracil (5-FU) was administered byintraperitonealinjection at 150 mg/kg 6-12 hours later. This schedule was repeated every 10 days to examine the protective effects of quizartinib from multiple cycles of 5-FU. The approach of quizartinib priming plus 5-FU every 10 days was examined as a treatment for a mouse model of AML.
Results
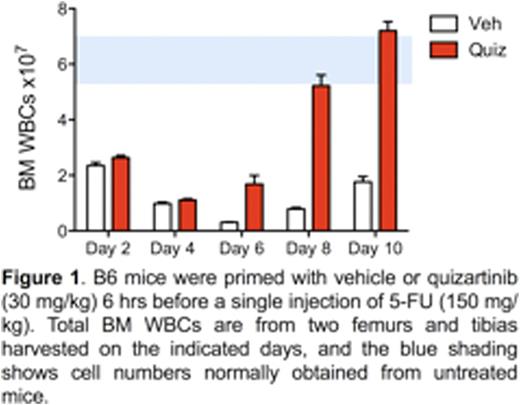
This work is based on studies from mice showing that a single dose of the FLT3 inhibitor quizartinib induces a rapid but transient quiescence of multipotentprogenitors (MPPs). MPPs are highly proliferative FLT3-expressing cells that are responsible for maintaining life-long blood production. They are markedly depleted by chemotherapy so their loss is a significant component of myelosuppression. We found that a single dose of quizartinib 6-12 hours before 5-FU resulted in a 10-fold increase in the survival of MPPs two days after administration. Quizartinib priming also provided significant protection to hematopoietic stem cells and committed progenitors within the lin-, Sca-1-, c-Kit+ population.Analysis of bone marrow at 2-10 days after a single 5-FU injection revealed the rapid recovery conferred to quizartinib-primed mice compared to mice primed with vehicle (Fig. 1).
To test whether quizartinib provided protection from multiple cycles of 5-FU we injected mice once every 10 days with 5-FU following a priming dose of quizartinib or vehicle 12 hrsearlier. This 10-day cycle of 5-FU is a potent regimen that causes bone marrow failure and lethality. We found that quizartinib priming promoted a marked level of protection, with only 1 of 5 mice dying over a period of 15 cycles, whereas all vehicle-primed mice succumbed between the 4th and 6th cycles. In addition, we found that quizartinib-primed mice did not lose weight and showed a rapid recovery of white and red blood cells and platelets.
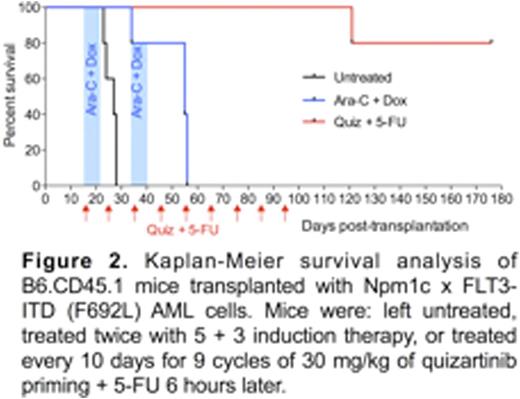
From these findings we hypothesized that quizartinib priming before chemotherapy may provide a safe and effective approach for treating cancers that are independent of FLT3signaling. We tested this with an AML model developed from Npm1c x FLT3-ITD(F692L) mutant mice. The F692L mutation confers resistance to quizartinib. The suitability of this model was confirmed from experiments showing that quizartinib dosing did not induce quiescence of Npm1c x FLT3-ITD(F692L) AML cells, nor did it protect them from 5-FU. A cohort of non-irradiated B6.CD45.1 mice transplanted with Npm1c x FLT3-ITD(F692L) AML cells was therefore established. At 16 days after transplantation, when AML cells were evident in the peripheral blood, the mice were divided into three groups: (i) untreated, (ii) induction therapy treated, and (iii) quizartinib primed + 5-FU treated.The induction therapy involved two rounds ofcytarabineat 50 mg/kg for 5 days and doxorubicin at 1.5 mg/kg for 3 days (i.e. 5+3 therapy). All mice in the untreated and induction chemotherapy-treated groups succumbed within 30 and 56 days respectively (Fig. 2). In contrast, only 1 of 5 mice treated by 9 cycles of quizartinib priming + 5-FU succumbed (at day 121). The remaining 4 mice were healthy at 176 days after transplantation, and no AML cells were detectable in their blood or BM. Furthermore, surviving mice had WBC counts within the normal range.
Conclusions
This work has shownthat priming mice with quizartinib provides significant protection to the hematopoietic system from 5-FU cytotoxicity. This has resulted in the development of a novel quizartinib + 5-FU treatment protocol for a preclinical model of AML whereby it markedly enhanced the survival of mice compared to mice receiving conventional therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal