Abstract
[Background]
Total body irradiation (TBI) has been thought to help donor cell engraftment in allogeneic hematopoietic cell transplantation (HCT) from alternative donors by killing recipientfs T cells. On the other hand, it might increase non-relapse adverse events due to induction or deterioration of inflammatory states following conditioning, leading in organ damages. However, the clinical significance of TBI may be changed by recent progress of HCT strategies, including donor selection algorithms, conditioning intensity, prophylaxis of graft-versus-host disease (GVHD), practical antibiotics usage during febrile neutropenia, and various supportive cares. Thus, the impact of TBI on neutrophil engraftment following HCT was retrospectively analyzed, using Japanese transplant registry database.
[Patients and methods]
We retrospectively analyzed 3933 adult recipients (>15 y.o.) who underwent HCT between 2006 and 2013 from 8/8 HLA-matched unrelated bone marrow donor (MUD, n=1367), HLA-mismatched unrelated bone marrow donor (MMUD, n=1102), and unrelated cord blood (UCB, n=1464). HCT using unrelated peripheral blood stem cells was excluded because it was currently too small in Japan. Only standard-risk leukemia patients were included: 1st or 2nd complete remission of acute myelogenous leukemia or acute lymphoblastic leukemia, 1st or 2nd chronic phase of chronic myeloid leukemia, and myelodysplastic syndrome other than refractory anemia with excess blasts. Conditioning regimens were divided into 5 groups: High-TBI (>=12Gy) myeloablative conditioning (MAC), fludarabine (Flu)-based Low-TBI (<=8Gy) MAC, no-TBI MAC, Flu-based Low-TBI (<=8Gy) reduced-intensity conditioning (RIC), and Flu-based no-TBI RIC.
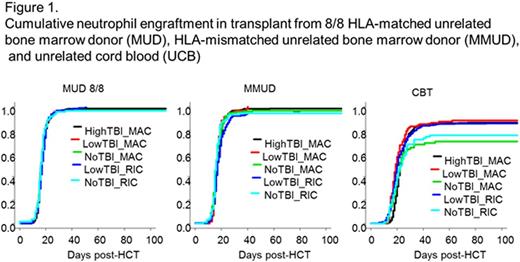
First, the impact of TBI on cumulative neutrophil engraftment was individually analyzed in overall MUD, MMUD, and UCB cohorts. Next, we evaluated the effects of TBI in UCB subgroups stratified according to conditioning intensity, number of HLA-mismatch, and the presence of anti-HLA antibodies.
[Results]
Neutrophil engraftment was sufficiently achieved among the High-TBI-MAC, Low-TBI-MAC, no-TBI-MAC, Low-TBI-RIC, and no-TBI-RIC groups both in MUD (>95% in all regimens at day 30) and MMUD (>92% in all regimens at day30) (Figure 1), and TBI was not significantly associated with prompt neutrophil engraftment in multivariate analyses. On the other hand, in UCB, no-TBI-MAC and -RIC groups had lower neutrophil engraftment compared with TBI-regimens (86% in High-TBI-MAC vs. 87% in Low-TBI-MAC vs. 70% in no-TBI-MAC vs. 85% in Low-TBI-RIC vs. 76% in no-TBI-RIC at day-60, P<0.001, Figure1). In multivariate analyses, TBI-regimens in UCB were significantly associated with successful neutrophil engraftment (HR 1.41, P=0.021 in High-TBI-MAC; HR 1.90 in Low-TBI-MAC, P<0.001; HR 1.57 in Low-TBI-RIC, P<0.01), after adjusting for age, gender, performance status, disease type, GVHD prophylaxis, use of in vivo T-cell depletion, infused cell doses, and use of granulocyte-colony stimulating factor. This beneficial effect on engraftment from TBI-based regimens was also observed in the UCB subgroups stratified according to conditioning intensity. Therefore, we subsequently focused only on HCT from UCB, and assessed the beneficial effect of TBI on neutrophil engraftment in the subgroups stratified according to number of HLA allele match, and the presence of anti-HLA antibodies.
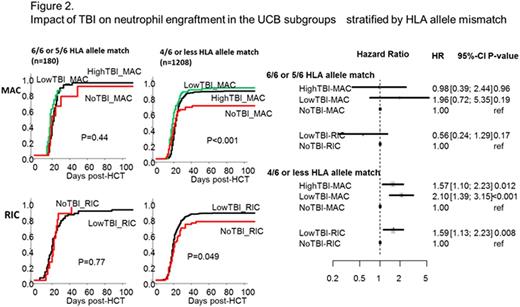
In the subgroups by number of HLA allele match, TBI-regimens were significantly associated with successful neutrophil engraftment only in recipients who received UCB with 4/6 or less HLA allele match, while the beneficial effect by TBI was not observed in those with 6/6 or 5/6 HLA allele match (Figure 2).
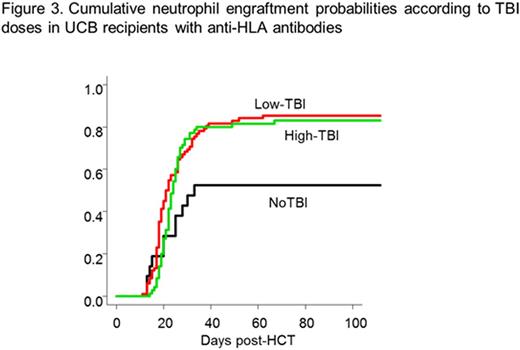
Additionally, focusing on recipients who had anti-HLA antibodies (n=173, Figure 3), TBI-based regimens were significantly associated with successful neutrophil engraftment (HR 2.38 in High-TBI regimens, P=0.017; HR 2.23 in Low-TBI regimens, P=0.016).
[Conclusion]
TBI-regimens had neither impact on neutrophil engraftment in the current practice of unrelated bone marrow transplantation. However, in UCB transplantation, TBI is still necessary to enhance engraftment, no matter what conditioning intensity is selected. Especially, recipients from UCB with two or more allele mismatches or with anti-HLA antibodies may benefit from adding TBI into conditioning.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal