Abstract
Introduction
The hematopoietic cell transplantation comorbidity index (HCT-CMI) at the time of transplant has previously been shown to predict the development of grade 3/4 acute GVHD (aGHVD) and higher non-relapse mortality (NRM) for patients with a high CMI (≥3). It has been postulated that inflammatory cytokines associated with many of the conditions accounted for in the CMI may contribute to GVHD development, severity, and ultimately mortality. Because of concerns for corticosteroid toxicities in frail or elderly patients, some clinicians will attempt to rapidly taper steroid doses. We hypothesized that patients with a high CMI and/or advanced age (>60 yo) are treated differently with respect to systemic steroids, and this results in a higher likelihood of progression to more severe aGVHD, increases the need for second-line GVHD therapy, and increases NRM. In order to test this hypothesis, we examined steroid tapering patterns in patients who received systemic steroids for aGVHD in order to determine the influence of age and/or CMI on the rate of taper, and whether this impacted treatment outcome.
Methods
Patients who underwent a first allogeneic HCT at MD Anderson Cancer Center between January 2010 and 2015 were retrospectively identified in our HCT database. All patients ≥18 years old that developed aGVHD in the first 100 days and required systemic therapy were evaluated. Detailed information on GVHD therapy, steroid tapering, indication for taper, response, and outcomes were collected retrospectively from the medical records using standard criteria. Competing risks analyses were used to estimate the incidence of second line therapy and NRM, and to evaluate predictors of early (≤5 days) taper, need for second line therapy, and non-relapse mortality. Incidence of second line therapy and NRM were estimated in landmark analysis starting on the date of initial taper.
Results
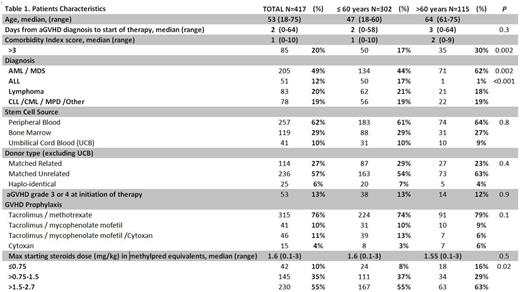
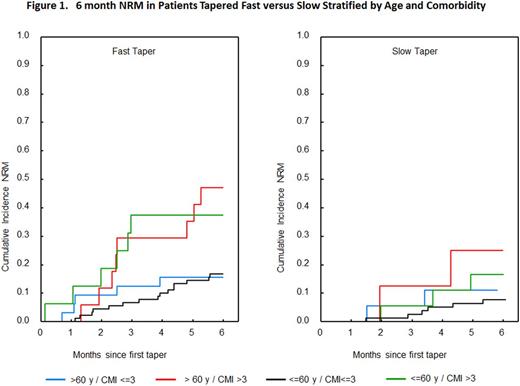
A total of 417 patients met the eligibility criteria for this analysis (Table 1). Median age of patients was 53 (range 18-75; 28% >60 yo), median CMI was 1 (0-10). Grade of aGVHD at the initiation of therapy was ≤ 2 in 87% of patients and grade 3/4 in 13% of patients with a median time to initiation of therapy of 2 days (0-58). The time from aGVHD diagnosis to initiation of therapy, initial aGVHD grade, and median starting steroid dose, (mg/kg/day) in methylpred equivalents, were comparable in patients ≤60 yo vs >60 yo (Table 1). However CMI>3 was higher in patients >60 yo as would be expected. The median time to initial taper was 5 days (1-26). Predictors for early taper (≤ 5 days) on multivariate analysis included CMI>3 in patients older than 60 yo (HR=1.6, p=0.02) and starting steroid dose >1.5 mg/kg/day (HR=1.5, p=0.001). In the subset of patients with grade 2-4 aGVHD (N=276) who were initially tapered for response, the median steroid dose on day 14 was significantly lower in patients >60 yo with a CMI>3 (0.4 [range 0.05-1.5] vs 0.7 [range 0-9]; p= 0.02) in all other patients. In responding patients, early taper was significantly predictive for cumulative incidence of second line therapy within the first month from the time of first taper (14% vs 6%, HR=2.5, p=0.04). The highest incidence of second line therapy (29%) was in patients >60 yo with CMI>3 who were tapered within the first 5 days (HR 2.8, CI 1.03-7.5, p=0.04). Importantly, none of the patients >60 yo with CMI>3 (n=8) who were tapered after 5 days required second line therapy (n=8). In patients >60 yo with CMI>3, those who were tapered early had a 6-month NRM of 47% vs 25% in those tapered after 5 days (p= 0.1).
Conclusions
These results confirm earlier reports implicating the impact of a high CMI at the time of transplant on aGVHD-related mortality. Our data offers evidence that tapering practices differ according to age and CMI and that this directly impacts GVHD outcomes. Investigating alternative (steroid sparing) strategies to prevent under-treatment of aGVHD is thus essential to improving survival outcomes in these patients.
Ciurea:Spectrum Pharmaceuticals: Other: Advisory Board; Cyto-Sen Therapeutics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal