Abstract
Background: Chimeric antigen receptor T-cell (CAR-T) therapy has shown significant efficacy in hematological malignancies, however, the efficacy against solid tumors remains limited. Immunosuppression caused by the tumor microenvironment or poor infiltration of transferred T cells can restrict T cell efficacy. We hypothesized that oncolytic Adenovirus (O-Ad) expressing cytokines would improve the efficacy of adoptive T cell therapy by modulating the tumor environment. Therefore we aimed to determine if O-Ad expressing cytokines can 1) cause direct lysis of pancreatic cancer cells, 2) enhance killing by CAR-T cells. 3) enhance infiltration and persistence of CAR-T cells in the context of solid tumors.
Methods: We targeted pancreatic tumor cell lines by mesothelin-redirected CAR-T cells (SS1-BBz CAR-T) in combination with O-Ad (Adv-5/3-d24-TNF-IL2), which consists of an adenovirus serotype 5 nucleic acid backbone, a serotype 5/3 chimeric fiber knob, a 24-bp deletion (d24) in the Rb binding constant region 2 of E1 promoter, an E2F tumor specific promoter and the human cytokines interleukin 2 and tumor necrosis factor alpha.
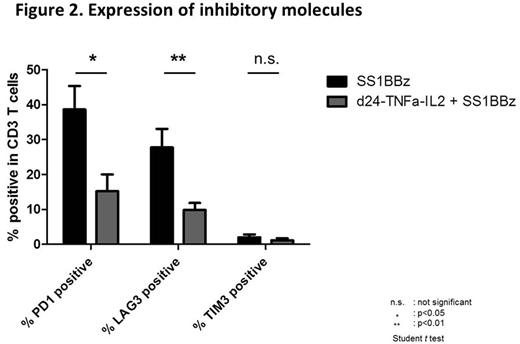
Results: The pancreatic tumor cell lines used in this study, ASPC1, BXPC3, and Capan2, expressed the Adv-serotype 3 receptor, DSG2. We also confirmed that O-Ad does not have adverse effects on T cell viability and proliferation even at high titer (1,000vp/cell) in an in vitro assay. To test the efficacy of the O-Ad and CAR-T combination, we performed a killing assay. O-Ad clearly enhanced killing by CAR-T cells in a luciferase based killing assay. We also used the xCELLigence real time cell analyzer (RTCA) for kinetic analysis of killing. In combination with O-Ad, more rapid killing kinetics by CAR-T cells was observed especially in lower E:T ratio. To examine the impact of the combination in vivo, large established subcutaneous ASPC1-CBG-GFP tumors in NSG mice were treated with CAR-T alone or in combination with intratumoral injection of O-Ad. O-Ad alone or CAR-T alone showed moderate tumor regression. On the other hand, the combination of O-Ad and CAR-T showed significantly enhanced tumor regression (figure 1). In FCM and histological analysis, tumors treated with O-Ad were infiltrated with higher number of T cells, and the number of infiltrating T cells correlated with anti- tumor efficacy. One of the concerns with use of IL-2 effects is the induction of regulatory T cells, however, there wasn't any difference in the number of CD25 and FOXP3 positive cell infiltration between the tumors treated with CAR-T alone and the combination of O-Ad and CAR-T. In a peripheral blood analysis, T cells from mice treated with the combination of O-Ad and CAR-T expressed lower levels of inhibitory molecules (PD-1, LAG3) comparing to those treated with CAR-T alone (figure 2).
Conclusions: These results suggest that combination therapy of O-Ad armed with cytokines and CAR-T cells is effective in preclinical models against solid tumors by enhancing T cell proliferation, persistence, function and infiltration to the tumor.
Scholler:Novartis: Patents & Royalties; University of Pennsylvania: Patents & Royalties: FAP-CAR US Patent 9,365,641 for targeting tumor microenvironment. Tähtinen:TILT Biotherapeutics Ltd.: Patents & Royalties: "Enhanced Adoptive Cell Therapy", PCT/EP2014/057776. Parviainen:TILT Biotherapeutics Ltd: Employment. Siurala:TILT Biotherapeutics Ltd: Employment. Hemminki:Targovax ASA: Equity Ownership; TILT Biotherapeutics Ltd.: Employment, Equity Ownership. June:Celldex: Consultancy, Equity Ownership; University of Pennsylvania: Patents & Royalties; Pfizer: Honoraria; Immune Design: Consultancy, Equity Ownership; Johnson & Johnson: Research Funding; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; Tmunity: Equity Ownership, Other: Founder, stockholder .
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal