Abstract
Transplantation of T cell-depleted BM (TDBM) under mild conditioning, associated with minimal toxicity and reduced risk of GVHD, offers an attractive therapeutic option for patients with nonmalignant hematologic disorders or can be used to induce immune tolerance to subsequent organ transplantation. However, overcoming TDBM rejection after reduced conditioning remains a challenge. We previously demonstrated that this barrier can be overcome using donor-derived naïve CD8 T cells if they are expanded against third-party alloantigens under culture conditions inducing a central memory phenotype. Thus, such anti-3rd party CD8 T cells (Tcm) exhibit marked veto activity and reduced risk for GVHD due to the lower frequency of anti-host clones achieved during the culture period. In the present study, we tested the feasibility of generating veto Tcm by stimulation against well-defined peptides including viral antigens. For proof of concept, we used OT1 mice that express a transgenic (Tg) TCR designed to recognize ovalbumin (OVA) residues 257-264 in the context of H2Kb MHC-I. Prior to harvest of OT1 CD8+ T cells, mice were immunized twice with OVA-peptide. Mice were sacrificed 7 to 14 days after immunization, their spleens and lymph nodes removed and crushed, and magnetic bead sorting utilized to isolate the memory cells (CD8+CD44+). The resulting population was subjected to third-party stimulation by co-culture with irradiated splenocytes generated from spleens of OVA-expressing mice, under cytokine deprivation. hIL-15 (10ng/ml) was added to the culture 60 hrs after culture initiation to induce the cells to express a Tcm like phenotype, as previously described for anti-3rd party Tcm generated from WT naïve CD8 T cells (WT Tcm).
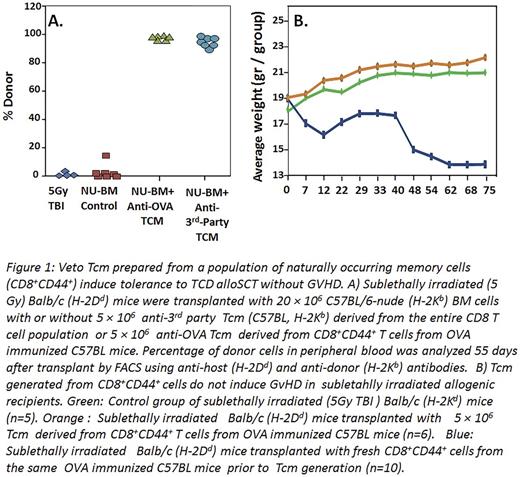
When tested in vivo, the OT-1 Tcm (H2Kb) were able to enhance engraftment of allogeneic T cell depleted BMT (H2Kb) in sub-lethally irradiated (5.5 Gy TBI) Balb/c recipients (H2Kd), in analogy to the chimerism induced by WT Tcm. This initial successful experiment was then followed by an experiment, which more closely resembled the human setting, in which the CD8+CD44+ cells were isolated from WT C57BL/6 OVA-immunized mice and subsequently introduced to co-culture with irradiated splenocytes generated from spleens of OVA-expressing mice. Results showed that Tcm grown from such a starting population of CD8+CD44+ cells were also able to achieve marked donor chimerism when administered with megadose of T cell depleted (TCD) alloSCT in sublethally irradiated Balb/c recipients (Fig.1A). Notably, infusion of 5x106 Tcm into sublethally irradiated mice (5.5. GY TBI) without BMT, did not cause any GVHD as measured by weight loss, in contrast to CD8+CD44+ T cells used to generate the Tcm (Fig.1B). These results demonstrate that CD8+CD44+ derived from memory CD8 T cells by expansion against cognate peptides exhibit markedly reduced risk for GVHD compared to freshly isolated memory cells, while retaining their veto activity and inducing tolerance.
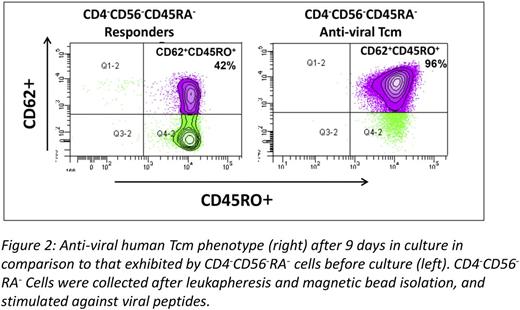
Finally, based on these proof of concept studies, we were able to translate this approach and generate human anti-viral CD8 veto cells with central memory phenotype. Thus, CD8+CD45RO+ memory T cells were selected by depletion of CD4+CD56+CD45RA+ cells from PBMC of normal donors and then co-cultured with donor DCs pulsed with a viral peptide mixture of three prominent viruses (EBV, CMV and Adenovirus). In three large scale experiments using leukapheresis preparations of normal CMV and EBV positive donors, with GMP grade reagents, more than 1x109 Tcm could be attained by the end of 9 days of culture (average expansion of Tcm=13.5±4 fold) with greater than 90% purity of CD45RO+CD62L+CD8+ T cells (Fig 2).
The harvested anti-viral Tcm exhibited more than 3 log depletion of alloreactivity, compared to fresh CD8 T cells, as measured by limit dilution analysis of cytotoxic T cell precursors against host type target cells (Fig. 3).
In conclusion, our results suggest that potent veto CD8 Tcm can be generated from the memory pool of donors positive for viral reactivity by stimulation against viral antigens. Such veto Tcm could be most attractive for haploidenitcal transplantation of TCDBM, as they can enable engraftment following non-myeloablative conditioning and at the same time provide anti-viral protection.
*E.B.L and N.O.G contributed equally
Bachar Lustig:Yeda LTD: Patents & Royalties. Or Geva:Yeda LTD: Patents & Royalties. Lask:Yeda LTD: Patents & Royalties. Gidron:Yeda LTD: Patents & Royalties. Kagan:Yeda LTD: Patents & Royalties. Reisner:Cell Source LTD: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal