Abstract
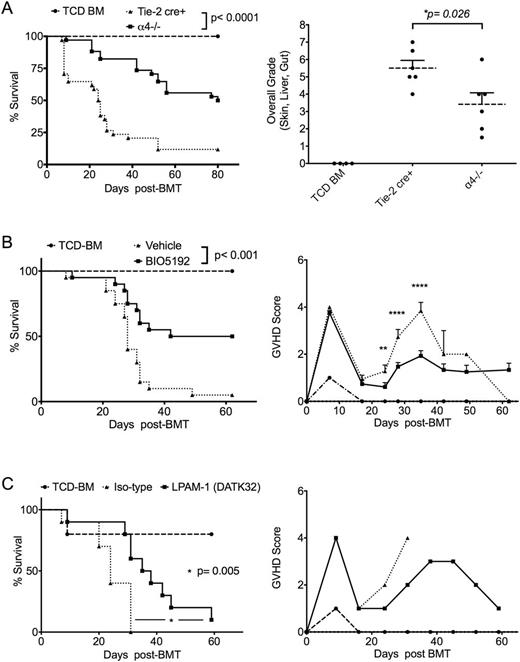
Allogeneic hematopoietic cell transplantation (allo-HCT) is considered a curative treatment for many malignant and non-malignant hematological conditions. However, treatment related mortality (TRM) limits the use of such treatment modality. Acute graft versus host disease (aGvHD) is responsible for more than 50% of TRM. Alloantigen presentation to donor T cells results in alloactivation of donor T cells and GvHD. Alloreactive donor T cells are also responsible for the beneficial graft versus leukemia (GVL) effect. Furthermore, alloactivation is also crucial for the development of immune tolerance. Thus, direct inhibition of alloreactive T cells might negatively impact GVL and the development of immune-regulatory cells. Modulation of alloreactive T cell trafficking and migration especially to the GI tract has been of great interest. α4 integrin plays a major role in transendothelial migration of immune cells to secondary lymphoid tissues. Thus, we hypothesize that α4 deficient donor T cells will fail to effectively traffic to GVHD target organs. To test this hypothesis, we generated Tie-2 cre+ α4fl/fl mice (B6, H-2b, CD45.2+) from which T cells were obtained. After in vitro activation of murine pan T cells using mixed lymphocyte reaction assay, we found α4 and β1 integrin are more highly expressed than β7 and αE integrin on WT T cell. Using (B6 to Balb/c) fully MHC mismatched mouse allo-HCT model we found that only donor derived alloreactive T cells (Ly5.1; derived from donor splenocytes) overexpressed α4 integrin but not bone marrow derived T cells (Ly5.2) at day +17 and +30 after transplant. As previously described (Alahmari B et al. 2015 ASH), we found that α4(-/-) T cells transplanted into allogeneic transplant recipients (B6 to Balb/c) resulted in significantly reduced acute GVHD and increased survival compared to recipients infused with WT T cells (Fig. A). We hypothesized that defective T cell transendothelial migration may contribute to less GvHD observed with α4(-/-) T cells. We performed bioluminescence imaging (BLI) to track CBRluc-transduced murine pan T cells (2 × 106 cells) after allo-HCT in vivo. We found a significant difference in the percentage of BLI signal intensity between α4 deficient and control T cells in spleen and gut at day 14 and 21 post allo-HCT. Using luciferase expressing mouse A20 leukemia cells (BALB) we found α4(-/-) T cells were able to clear leukemia cells as effectively as WT T cells. Of interest in vitro activation of T cells using anti-CD3/CD28 antibody-coated beads resulted in significantly increased expression of CTLA-4 expression on α4(-/-) compared to WT T cells. In spite of this, in vivo blockade of CTLA-4 after allo-HCT did not accelerate the onset or increase the severity of GVHD in mice receiving α4(-/-) T cells compared to recipients receiving WT T cells, suggesting that the protective effect of α4(-/-) T cell is independent of T cell immunoregulatory function. α4 integrin can form a heterodimer with either β1 to form α4β1 integrin (VLA-4) or β7 to form α4β7 (LPAM-1). Previous results of others (Petrovic A et al. 2004 Blood; Waldman E et al 2006 Blood) implicated β7 integrin in aGvHD, especially GI GvHD. To define which α4 heterodimer (α4β1 or α4β7/αEβ7) is the more potent in GVHD induction, we performed (B6 to Balb/c) allo-HCT and treated mice with either BIO5192 (a highly selective VLA-4 inhibitor) from day +2 to + 28, or with LPAM-1 monoclonal antibody (DATK32) 3 times per week for one month. Although both interventions resulted in reduced GVHD and increased survival, treatment with BIO5192 appeared to be more effective as an approach to reduce GvHD (Fig. B and C).
In conclusion, α4 integrins is involved in GVHD pathology likely through facilitating the migration of alloreactive T cells to GVHD target organs (especially the GI tract). In addition, we report for the first time that selective inhibition of α4β1 integrin (VLA-4) is more effective than that of α4β7 (LPAM-1) in prevention of GVHD in a fully MHC mismatched allo-HCT model. Blockade of α4 integrins (natalizumab, vedolizumab) especially α4β1 should be considered a valid approach to treat or prevent GvHD in humans and can be exploited to treat other inflammatory settings such as solid organ transplantation, chronic inflammatory diseases and autoimmune diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal