Abstract
Introduction: Histone deacetylase inhibitors (HDACis) have demonstrated clinical efficacy in multiple myeloma, particularly in combination with proteasome inhibitors. CHR-3996 is a class 1 selective HDACi with potent anti-myeloma activity in vitro. Aminopeptidase inhibitors act downstream of the proteasome and prevent breakdown of proteasome generated peptides into amino acids. Synergistic cytotoxicity was observed in vitro when CHR-3996 was combined with the aminopeptidase inhibitor, tosedostat through rapid activation of NFkB followed by increased expression of the repressors IκBα, A20, CYLD, BIRC3. The MUK-three study was designed to translate these pre-clinical findings into a phase 1 clinical trial. This dose escalation study aimed to determine the maximum tolerated dose, safety and preliminary activity of CHR-3996 administered in combination with Tosedostat for patients with relapsed, refractory MM. Here we present the final study results.
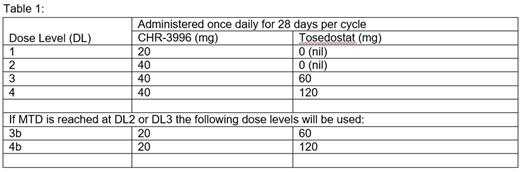
Methods: MUK-three was an open label multi-centre UK Phase I/IIa trial for patients with relapsed and relapsed/ refractory myeloma who had failed conventional treatments. Patients were permitted to meet the haematological entry criteria using growth factor and/or blood product support. During dose escalation subjects received CHR-3996 (20-40mg days1-28) and Tosedostat (0-60mg days 1-28) (Table 1) every 28 day cycle until disease progression or withdrawal. Dose limiting toxicities (DLTs) were evaluated during cycle 1 and dose escalation followed the 3+3 design. Responses were assessed using modified IMWG uniform response criteria, with the primary endpoint for the expansion phase of stable disease (SD) rate after 4 cycles of therapy. Toxicity was graded by CTCAE V4.0.
Results: The trial was open to recruitment from July 2012 to December 2015. 20 patients were treated during dose escalation, including 8 at the recommended dose (RD) and 12 at dose levels (DL) 1-3. Only 1 DLT was observed at DL3 (grade 4 thrombocytopenia); however, this DL was deemed not tolerable due to the high incidence of low grade gastrointestinal toxicities. Hence the RD was determined as DL3b, CHR-3996 20mg and Tosedostat 60mg. A further 2 patients were treated at RD during dose expansion to make the required 10 patients for the protocol defined initial analysis at which point the trial closed.
At the RD (n=10) median age was 63 years (range 47-73). 80% of patients had received at least 4 prior lines of therapy (median 4, range 2-9); 50% were ISS II, 30% ISS III; 4/6 patients with evaluable FISH data had 1q gain. The median time from diagnosis to treatment for the overall population was 85.3 months (27.5-198.8). The median number of cycles received was 2.5 (range 2-8) and 2 patients remain on treatment with 8 stopped due to disease progression. The 2 patients ongoing (received 5 & 9 prior lines) had their schedule adjusted to a 5 day a week dosing to further improve tolerability. Both had a clinical response (1MR, 1PR) and remained progression free at 6 months.
3/10 patients had SD after 4 cycles, the overall response rate (≥PR) was 1/10(10%) and the clinical benefit rate (≥MR) 2/10 (20%). Overall outcomes were: PR 10%, MR 10%, and SD 30%. Median time to maximum response was 1.84 months (95% CI [1.09, 8.65]).
Toxicities at the RD were manageable, 30% of patients required a dose reduction. 22 serious adverse events were reported in 16 patients across all doses, mainly infections (10/22, 45.5%). The commonest grade 3-4 toxicities reported for all 22 patients were: platelet count decrease (12, 54.5%), white blood cell decreased (6, 27.2%), diarrhoea (5, 22.7%). The most frequent grade 1-2 toxicities were fatigue (15, 68.2%), nausea (14, 63.3 %), anorexia (14, 63.6%), anaemia (13, 59.1%). 1 patient withdrew due to toxicity, and there were no treatment related deaths.
Conclusions: This study demonstrated that the novel combination of CHR-3996 and tosedostat was safe and tolerable in multiply relapsed, refractory myeloma patients many of which had poor bone marrow function. The recommended dose of the combination was 20mg and 60mg, respectively. Following further adjustment to an intermittent 5 day/ week dosing schedule, treatment was well tolerated and clinical benefit observed. This suggests that further evaluation of this novel combination is warranted.
Acknowledgments: This trial was part of the Myeloma UK Clinical Trial Network, ISRCTN: 24989786.
Williams:Novartis: Honoraria; Janssen: Honoraria, Other: Travel support, Speakers Bureau; Celgene: Honoraria, Other: Travel support, Speakers Bureau; Takeda: Honoraria, Other: Travel support, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Yong:Autolus Ltd: Equity Ownership, Patents & Royalties: APRIL based chimeric antigen receptor; Janssen: Research Funding. Cook:Takeda Oncology: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Glycomimetics: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau. Jenner:Amgen: Consultancy, Honoraria, Other: Travel support; Takeda: Consultancy, Honoraria, Other: Travel support; Janssen: Consultancy, Honoraria, Other: Travel support, Research Funding; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding. Morgan:Univ of AR for Medical Sciences: Employment; Bristol Meyers: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Davies:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal