Abstract
Introduction: Multiple myeloma is a heterogeneous plasma cell neoplasm that remains all but incurable despite significant advances in treatment. We anticipate that the ability to overcome this hurdle resides in personalized strategies designed to specifically recognize, target, and anticipate dynamic tumor subpopulations with variable drug response profiles within an individual. To this end, we have developed a novel multi-disciplinary approach using organotypic drug screening and mathematical modeling to assess drug sensitivity of the different subpopulations within the tumor burden of individual patients and, in turn, provide accurate predictions of clinical outcome to anti-myeloma therapy.
Material and methods: We have used a novel combination of ex vivo drug sensitivity assay and mathematical models to predict clinical response of 48 MM patients (11 newly diagnosed and 37 relapsed, 18 females and 30 males, median age 64.5, range 45-77) treated with a combination of proteasome inhibitors and IMIDs (37), nuclear export and topo2 isomerase inhibitors (10), and high dose melphalan (1). MM cells (CD138+) were extracted from fresh bone marrow aspirates and seeded in an ex vivo co-culture model with human stroma in 384-well plates. These cells were exposed to a number of chemotherapeutic and experimental agents (up to 31) for a period of 4 days, during which viability was assessed continuously using bright field imaging and digital image analysis. A mathematical model was used to interpolate the dose response dynamics to each drug, and combined with drug and regimen-specific pharmacokinetic data, generate predictions of clinical response to each individual drug. We have then validated ex vivo-based predictions with actual outcome 90 days post-biopsy. In patients treated with combinations, the mathematical model combined the effect of each single drug assuming additivity.
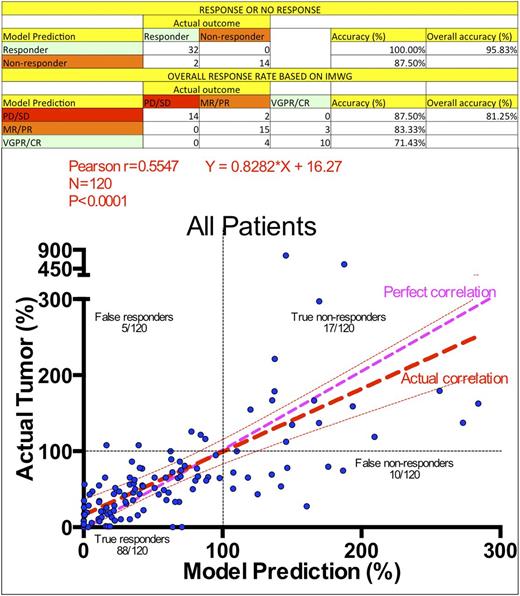
Results: To examine the accuracy of the predicted in silico responses, we have assessed the model according to three increasingly strict standards of accuracy: (A) The model correctly predicted 32 out of 32 responders (100%) and 14 out of 16 non-responders (88%), with an overall accuracy of 96%; (B) According to IMWG stratification, the model correctly stratified 14 out of 16 patients as stable or progressive disease (PD/SD, 88%, the remaining 2 incorrectly predicted as MR/PR), 15 our of 18 as minimal or partial response (MR/PR, 83%, the remaining 3 incorrectly predicted as VGPR/CR), and 10 out of 14 patients as very good partial response or complete response (71%, the remaining 4 incorrectly classified as MR/PR), with an overall accuracy of 81%; (C) The 48 patients from this study provided a total of 120 measures of tumor burden (M-spike or SFLC) within the 90-day post-biopsy period. The direct correlation between tumor burden measures and model predictions led to a Pearson r=0.5547 (P<0.0001) and the correlation line [Actual]=0.8282*[Model]+16.27, where [Model] and [Actual] are the predicted and actual tumor burdens as a percentage of tumor burden measure at the moment of treatment initiation. From the 14 non-responders, the model predicted that 2 would have had a VGPR/CR and 3 would have had a MR/PR if treated with drugs differing from those given clinically. Intriguingly, from the 13 patients who received 3-agent therapies with matched in silico drug testing, only 2 had response to all agents, 6 had a response to 2 agents, 4 responded to only 1 of the 3 agents and 1 responded to none of the agents. Next, we examined the 18 patients with a 2-drug match between in silico and actual treatment. From these, 1 patient model responded to 2 drugs, 12 responded to only 1 drug, and 5 responded to none. From the 17 patients with a single agent match between treatment and in silico, 8 had a response and 9 had no response. In summary, among the drugs tested both ex vivoand actually administered to patients, 48% had some predicted clinical benefit, while 52% of agents had none, and could theoretically be removed without affecting clinical outcome.
Conclusion: We observed an excellent correlation between in silicopredicted and clinically observed responses in 48 MM patient specimens. Our data suggest that this model may provide critical insight in the selection the appropriate therapeutic agents and number of agents to combine for a given individual. Further validation is required to better define the role of this approach as a clinical decision support tool.
Baz:Novartis: Research Funding; Signal Genetics: Research Funding; Karyopharm: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda/Millennium: Research Funding; Bristol-Myers Squibb: Research Funding; Merck: Research Funding. Shain:Signal Genetics: Research Funding; Takeda/Millennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen/Onyx: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal