Abstract
Recent evidences suggests that the efficacy of Lenalidomide (LEN) depends upon its ability to degrade IKZF1 and IKZF3 proteins via cereblon dependent ubiquitin proteasome pathway [Science. 2014 Jan 17; 343(6168): 301-305]. Based on this model it would theoretically be antagonistic to combine LEN with proteasome inhibitors (PI). However, it is well recognized that there is significant synergism when LEN is combined with PI and this combination is routinely and effectively used in the clinic. The mechanism of synergy and the fate of IKZF1 and IKZF3 when these two agents are combined is poorly understood. We undertook a series of experiments to study the fate of IKZF1 when this combination of drugs was used in multiple myeloma cells.
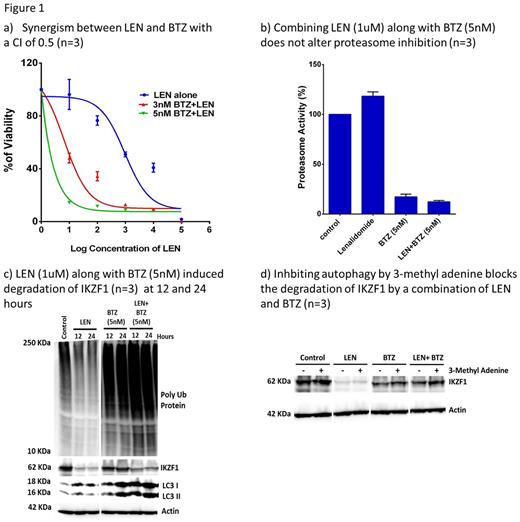
Combining LEN (1uM) along with bortezomib (BTZ; 1nM) a PI showed a significant kill on U266 cells (myeloma cell line) on day 5 post treatment (n=3; P=0.02) when compared to either of the agents alone. In an MTT assay, the synergism was well documented with a combination index of 0.5 (Fig 1a). Next we assessed the function of proteasome (chymotrypsin activity) when LEN was combined with PI. We observed that LEN alone does not interfere with proteasome activity. It was noted that BTZ alone at the concentration used (5 nM) was able to effectively inhibit the activity of proteasome (Fig 1b). It was also observed that combining these two agents does not interfere with BTZ action in inhibiting proteasome complex (Fig 1b). As a result of efficient proteasome inhibition, we observed an accumulation of ubiquitinated proteins in the BTZ and LEN + BTZ treated cells when compared to control and LEN alone treated cells (Fig 1c).
Next, we looked for the fate of IKZF1 in U266 cells treated with LEN, BTZ and in combination of both the drugs. As reported, we observed a degradation of IKZF1 in U266 cells upon treatment with LEN. While we did not see any degradation of IKZF1 in BTZ alone treated cells. It was noted that in combination treated cells (LEN+BTZ) there was a degradation of IKZF1 (Fig 1c). In spite of significant proteasome complex inhibition, degradation of IKZF1 was observed which suggested a proteasome independent mechanism. It is well known that proteasome inhibition results in upregulation of the autophagy pathway which in turn can degrade the accumulated ubiquitinated proteins. We noted that upon treatment with BTZ or LEN+BTZ an induction of autophagy was observed, as evidenced by an increase in generation of LC3II bands on an immunoblot (Fig 1c). To support our hypothesis that IKZF1 is degraded by autophagy in the absence of proteasome complex, we pre-treated the U266 cells with an autophagy inhibitor (3-methyladenine) followed by treatment with LEN and BTZ and noted an accumulation of IKZF1 proteins (Fig 1d). We also observed a downregulation of IKZF1 target genes IRF4 and c-MYC by 12 and 24 hours in the combination treated cells (data not shown). Taken together this data demonstrates that there is (i) significant in-vitro synergism between the two agents (ii) the combination additively induces autophagy pathway (iii) IKZF1 protein can be degraded via this autophagy pathway in the presence of effective proteasome inhibition. While additional mechanisms of synergy between these two agents cannot be excluded, further enhancing autophagy pathway in these cells by drugs like sirolimus (autophagy inducer in myeloma cells) could potentially improve the synergy between these two drugs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal