Abstract
The immunomodulatory drugs (also known as IMiDs) thalidomide, lenalidomide and pomalidomide, play a pivotal role in the treatment of multiple myeloma (MM). Recent studies have deciphered the mechanism of action of these drugs and shown that they directly interact with cereblon (CRBN), which is part of an E3-ubiquitin ligase complex. The IMiD-CRBN interaction selectively alters the affinity of CRBN to two important transcriptional factors, Ikaros (IKZF1) and Aiolos (IKZF3), leading to their ubiquitination and subsequent proteasomal degradation, representing a novel mechanism of therapy through ubiquitin-mediated alteration of gene expression.
Several studies have underlined the importance of CRBN for the biologic activity of IMiDs, as both human cell lines and patient samples with acquired IMiD-resistance, exhibit a significant reduction of CRBN expression. However, it still remains unknown how CRBN expression is regulated, and subsequently, whether the acquired resistance to IMiDs is potentially druggable and reversible.
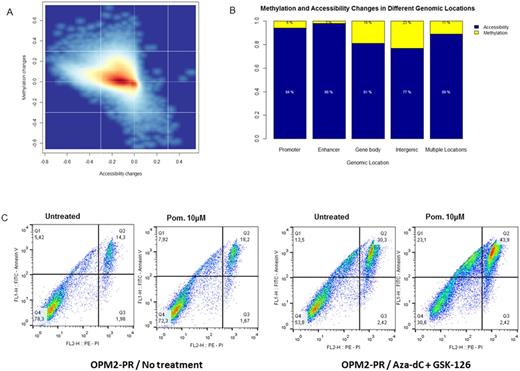
In this study, we have used the IMiD-sensitive human myeloma cell lines OPM2 and NCI-H929, which were cultured with subtoxic doses of Lenalidomide and Pomalidomide for 4-6 months for acquisition of stable resistance. The lenalidomide- and pomalidomide-resistant cell lines (OPM2-LR, NCI-H929-LR and OPM2-PR, NCI-H929-PR subsequently) were then used as a model of acquired IMiD-resistance. In line with previous studies, we confirmed a significant reduction of CRBN expression, but not it downstream targets, IKZF1 and IKZF3, in the resistant cell lines, both at mRNA and protein level. Using methylation-specific melting curve analysis, we next showed that the downregulation of CRBN is not due to promoter DNA methylation. We therefore proceeded with the assessment of chromatin accessibility through nucleosome positioning using AcceSsIble. Our data show that acquired IMiD-resistance is accompanied by increased nucleosome occupancy (and thus decreased chromatin accessibility - figure 1A) rather than DNA methylation, an event that is also more prominent in promoters and enhancers (figure 1B). Among the top 100 genes whose promoters are nucleosome-occupied was CRBN, an event that could explain the reduced expression observed upon acquired IMiD-resistance.
Since both DNA methylation and nucleosome positioning are two potentially reversible epigenetic modifications, we next treated the resistant cell lines with a combination of decitabine (a DNA-methyl-transferase inhibitor) and GSK-126 (an EZH2-inhibitor) for 3 days and then exposed them again to IMiDs. Even though the combination treatment by itself was mildly toxic for the cells, cell viability was even more reduced upon treatment with IMiDs three days after exposure to this combination of epidrugs, suggesting that epigenetic reprogramming and resensitization of IMiD-resistant cells could be feasible (figure 1C).
Taken together, our data could potentially explain the mechanism behind CRBN downregulation observed upon IMiD-resistance and provide evidence for possible epigenetic resensitising myeloma cells to IMiDs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal