Abstract
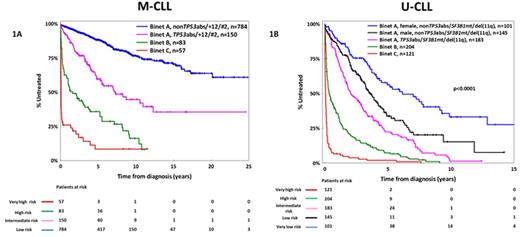
The classification of CLL patients according to the somatic hypermutation status (SHM) of the immunoglobulin heavy variable (IGHV) genes, namely mutated (M-CLL) versus unmutated (U-CLL), reflects fundamental differences in disease biology and clinical course. Realizing this, here we followed a compartmentalized approach and addressed the issue of prognostication separately for M-CLL and U-CLL. In a multi-institutional cohort of 2366 patients [M-CLL, n=1364 (58%); U-CLL, n=1002 (42%)] consolidated within ERIC, the European Initiative in CLL, we assessed the clinical impact of 'traditional' (age and clinical stage at the time of diagnosis, gender, CD38 expression, FISH detected abnormalities included in the Döhner hierarchical model of cytogenetic aberrations), and novel prognosticators (recurrent mutations within the TP53, SF3B1, NOTCH1, MYD88, and BIRC3 genes; IGHV gene usage; membership in stereotyped subsets) within M-CLL and U-CLL. Our statistical approach was based both on Cox regression models and recursive partitioning algorithms; internal validation was performed via bootstrapping procedures. Given the retrospective nature of our study, time-to-first-treatment (TTFT) was the primary endpoint. As expected, M-CLL exhibited significantly longer TTFT compared to U-CLL [median TTFT: not yet reached (M-CLL) vs 1.9 years (95% CI: 0.01-12.3 years, U-CLL), p<0.0001]. Advanced clinical stages (Binet B-C) were associated with shorter TTFT in both M-CLL and U-CLL; a significantly worse outcome was also identified for Binet C versus Binet B cases (p<0.0001). Binet A patients received our special focus, representing 90% and 67% of M-CLL and U-CLL studied cases, respectively. Amongst Binet A M-CLL cases, TP53 aberrations [TP53abs, deletions of chromosome 17p, del(17p) and/or TP53 mutations], stereotyped subset #2 membership and trisomy 12 were identified as equally adverse prognostic indicators [median TTFT: 5.5 (95% CI: 0.2-12.8), 4 (95% CI: 0.6-6.8) and 7.3 (95% CI: 0.7-13.4) years, respectively; p-value: non-significant when applying the log-rank test for all paired comparisons); of note, TP53abs were mutually exclusive with the other two features. Amongst Binet A U-CLL cases, TP53abs, SF3B1 mutations and deletion of chromosome 11q [del(11q)] had an overall similar adverse impact [median TTFT for TP53abs, SF3B1 mutations and del(11q): 1.8 (95% CI: 0.01-4.4), 2 (95% CI: 0.01-7.7) and 2.1 (95% CI: 0.01-8.1) years, respectively, p-value: non-significant when applying the log-rank test for all paired comparisons]. Within the remaining Binet A U-CLL cases [i.e. those lacking TP53abs and/or SF3B1 mutations and/or del(11q)], the only parameter associated with shorter TTFT was male gender (median TTFT: 3.5 years, 95% CI: 0.5-8.1 years). Based on these findings, we developed two prognostic indices for assessing TTFT tailored specifically to M-CLL and U-CLL, respectively. Within M-CLL (Figure 1A), 4 subgroups were identified: (i) very high risk: Binet C with identical 5- and 10-year treatment-probability (TP) of 92%; (ii) high risk: Binet B, 5y-TP and 10y-TP: 64% and 84%, respectively; (iii) intermediate risk: Binet A with one of the following: TP53abs or +12 or subset #2 membership, 5y-TP and 10y-TP: 40% and 55%, respectively; and (iv) low risk: Binet A nonTP53abs/+12/subset#2, 5y-TP and 10y-TP: 12% and 25%, respectively. Within U-CLL (Figure 1B), 5 subgroups were identified: (i) very high risk: Binet C with 5- and 10-year TP of 100%; (ii) high risk: Binet B, identical 5y-TP and 10y-TP: 90% and 100%, respectively; (iii) intermediate risk: Binet A with one of the following: TP53abs or SF3B1 mutations or del(11q), 5y-TP and 10y-TP: 78% and 98%, respectively; (v) low risk: Binet A, male nonTP53abs/SF3B1mut/del(11q), 5y-TP and 10y-TP: 65% and 85%, respectively and (iv) very low risk: Binet A, female nonTP53abs/SF3B1mut/del(11q), 5y-TP and 10y-TP: 45% and 65%, respectively. In conclusion, we identified clinicobiological parameters with distinct prognostic implications for M-CLL and U-CLL. These parameters were used in order to develop prognostic indices tailored to SHM status that were found capable of distinguishing subgroups with markedly different outcomes. We argue that such a compartmentalized approach may supersede previous attempts, thus overcoming the pronounced heterogeneity of CLL and optimizing prognostication.
PB and TM contributed equally as first authors
Rosenquist:Gilead Sciences: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal