Abstract
Background:
The Philadelphia chromosome (Ph) is the hallmark of chronic myeloid leukemia (CML). However a few patients present without Ph by conventional G-banding but express the BCR/ABL1 rearrangement detectable by molecular FISH and/or PCR representing a cryptic Philadelphia chromosome (i.e., Ph-, BCR-ABL1+ CML). There is a paucity of data regarding the outcome this small but important subset of patients when treated with tyrosine kinase inhibitors (TKI). In this report, we report the clinical characteristics and treatment outcome of these patients.
Methods:
We reviewed the medical records of all 630 patients with CML in chronic phase treated at a single institution in consecutive or parallel clinical trials with TKI as initial therapy between 2000 and 2015. Nine patients with Ph-negative BCR-ABL1-positive disease were identified. We analyzed their clinical characteristics and clinical outcome.
Results:
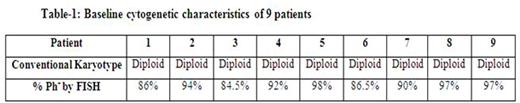
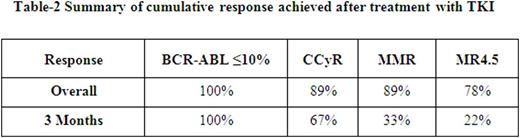
Median age of this group of patients was 34 years (range, 23 to 69); only 1 patient was aged >50 years. This is in contrast to the median age of 53 years for patients with standard Ph-positive CML. Male to female ratio was 1.2. Baseline cytogenetic characteristics are shown in Table-1. Transcript type was e14a2 in 4, e13a2 in 4, and both in 2. Eight patients presented with low risk Sokal score and one with high-risk score. Four patients received initial therapy with imatinib (400 mg daily in 1, 800 mg daily in 3), 2 with dasatinib, 2 with nilotinib and one with ponatinib. The cumulative response is presented in Table-2.
All patients achieved complete hematological response (CHR) within 3 months. Eight patients achieved complete cytogenetic response (CCyR), 6 of them within 6 months from start of therapy; 7 patients achieved MMR within 12 months from the start of therapy. MR4.5 was achieved in 7, and it has been sustained for at least 2 years in 5 patients; no patient has electively discontinued therapy.
After a median follow-up of 79 months from start of therapy, 5 patients remain on their original TKI (including the 2 patients who started on imatinib 800 but currently receiving imatinib 400 due to dose reduction) with the molecular response at last follow-up being undetectable BCR-ABL1 in 4 and MMR in 1. One patient transformed to blastic phase. Median overall survival (OS) of these patients was not reached (two patients died from unrelated causes: one from a car accident, the other, who had transformed to blastic phase, received therapy with clofarabine, idarubicin, ara-C then imatinib followed by SCT, and died from a fall); the other 7 patients are alive 13, 11,7.8, 6.5, 5, 4, and 3.4 years from diagnosis.
Conclusion:
Ph-negative BCR-ABL1-positive CML presents at a younger age than the average CML population, and usually presents with low-risk disease. These patients respond well to therapy with TKI and have a very favorable long-term outcome.
*One patient did not have molecular response assessment since he PCR testing was not routinely done before CCyR in 2001 when the patient had the 3 month response assessment.
Kantarjian:Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. DiNardo:Abbvie: Research Funding; Novartis: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Celgene: Research Funding; Agios: Other: advisory board, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal