Abstract
Background: Ponatinib is a potent oral tyrosine kinase inhibitor (TKI) active against native and mutant forms of BCR-ABL. It is approved for patients with resistant or intolerant CML or Ph+ ALL, or with the T315I mutation, which renders other TKIs ineffective. The USPI recommends a starting dose of 45 mg/day, with consideration of lower starting doses in patients with selected comorbidities. In the US, ponatinib is available exclusively through a specialty pharmacy that maintains prescribing data for all US ponatinib-treated patients. Analyses of dispensed ponatinib prescriptions for CP-CML patients show that median therapy duration exceeds 1.5 years but is variable across groups; examination of demographic, clinical and physician characteristics may reveal predictors of therapy duration.
Methods: We performed a retrospective analysis of patients starting treatment with ponatinib over the 2.5-year period from 01 January 2014 and 30 June 2016 using data from referring physicians, patient intake forms and pharmacy dispensing records. Gender (M/F), age (</≥65 years), reported T315I status (Y/N), line of therapy (2nd-5th), most recent prior TKI (imatinib, nilotinib, dasatinib, or bosutinib), starting dose (15, 30 or 45 mg/day), prescribing physician practice type (academic/community), and number of ponatinib patients treated by physician (1, 2, 3 or more) were examined as predictors of therapy duration using Kaplan-Meier (K-M) techniques and log-rank tests; multivariable, stepwise, proportional-hazard regression was used to generate adjusted hazard ratios and identify primary drivers of therapy duration.
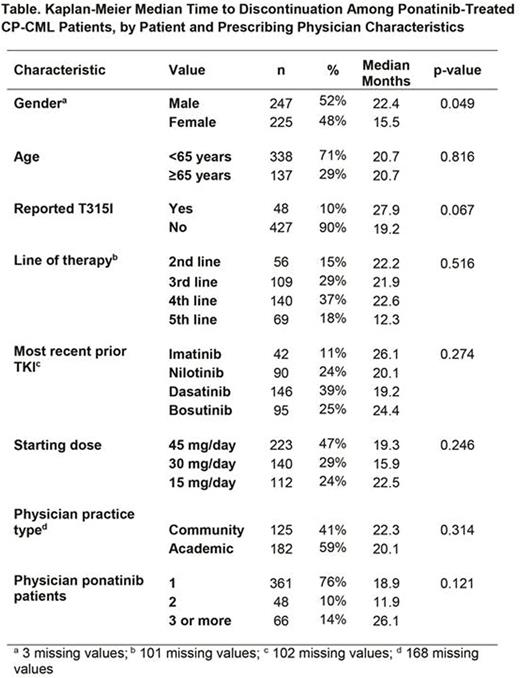
Results: 475 US patients identified with CP-CML initiated treatment with ponatinib over this 2.5-year period; K-M median time on therapy was 20.7 months. About one-half of patients were male (Table) and most were aged <65 years. Only 10% were reported to have the T315I mutation, most were in their 3rd or 4th line of therapy, and had most commonly switched from dasatinib; nearly one-half had a starting dose of 45 mg/day. Slightly more than one-half of prescribing physicians were in academic settings, and more than three-quarters had only one ponatinib patient. When examining each predictor individually, only gender was a significant predictor of time on therapy (Table), with women having significantly shorter duration than men. There was also a trend for T315I patients to have longer duration than non-T315I. In analyses adjusted for other covariates, non-T315I patients had significantly shorter duration (hazard ratio [HR]: 2.09; p=0.033), as did those whose most recent TKI was not imatinib (HR nilotinib v imatinib: 3.61; dasatinib v imatinib 4.72; bosutinib v imatinib 1.85; overall p=0.001). Women had a borderline significantly shorter duration than men (HR: 1.40; p= 0.085) and versus 2nd line, patients in 3rd (HR: 0.67) and 4th (HR: 0.68) line had longer duration, while 5th line had shorter (HR: 1.45; overall p=0.054).
Conclusions: Real-world US data for CP-CML patients receiving ponatinib show that a number of subgroups have median duration on ponatinib exceeding 2 years, but suggest drivers of therapy duration may be complex including both disease (e.g. T315I, prior line of tx) and patient (e.g. sex) related factors.
Study sponsor: ARIAD Pharmaceuticals, Inc.
Mauro:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. McGarry:ARIAD: Employment, Equity Ownership. Lustgarten:ARIAD: Employment, Equity Ownership. Huang:ARIAD: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal