Abstract
Background: In chronic phase (CP) chronic myeloid leukemia (CML) the disease characteristics at diagnosis, risk distribution, probability of transformation to accelerated/blast phase (AP/BP), and toxicities may vary according to age. Nilotinib has shown better efficacy compared to imatinib, but it has been associated to a higher incidence of arterial thrombotic events (ATEs). Little is known on the efficacy, safety and long-term outcome of nilotinib treated patients in different age groups.
Aims: To investigate the efficacy and safety, particularly the cardiovascular safety, of first-line treatment with nilotinib according to age distribution.
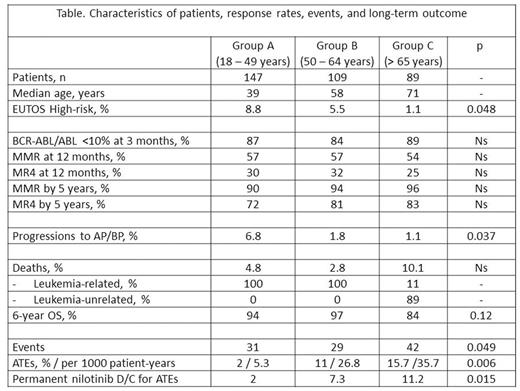
Methods: We analyzed 345 patients ≥ 18 years of age with CP CML enrolled in clinical trials of the GIMEMA CML WP investigating nilotinib as first-line treatment. Patients were treated with: nilotinib 400 mg BID (n=73); rotation of nilotinib 400 mg BID / imatinib 400 mg OD (3-month periods for each drug)(n=123); nilotinib 300 mg BID (n=149). The median follow-up was 58 months. We divided the patients in 3 groups of age (table): 18-49 years (group A, 147 pts, median age: 39 years); 50-64 years (group B, 109 pts, median age 58 years); and ≥ 65 years (group C, 89 pts, median age 71 years). We analyzed in detail the response rates, events and outcome. Definitions: Major molecular response (MMR): BCR-ABL≤0.1% (IS), with > 10.000 ABL copies; MR4: BCR-ABL≤0.01% (IS), with > 10.000 ABL copies. Events: permanent discontinuation of nilotinib for any reason, including adverse events, progression to AP/BP, or deaths. ATEs: peripheral arterial obstructive disease (PAOD), acute coronary syndrome, chronic ischemic heart disease, significant carotid stenosis and ischemic stroke, or other significant ischemic events.
Results: EUTOS high risk patients were: 8.8, 5.5, 1.1% in group A, B, and C, respectively (p=0.048). We did not observe significant differences in molecular response rates (table), including BCR-ABL/ABL <10% at 3 months, MMR and MR4 at 12 months, and cumulative incidences by 5 years of MR3 and MR4. Events leading to permanent nilotinib discontinuations occurred in 31%, 29%, and 42% of group A, B, and C, respectively (p=0.049). Overall, 29 ATEs were recorded, with higher rates, as expected, in elderly patients (group A: 2%; B: 11%; C: 15.7%; p=0.006); relative risk for ATEs in group B vs. A: 5; relative risk in group C vs. A: 6.7. ATEs were the reason for permanent nilotinib discontinuation in 2% of pts 18-49 years, 7.3% of pts 50-64 years, and 11.2% of pts > 65 years (p=0.015). Progressions to AP/BP were significantly more frequent in younger (18 - 49 years) patients (6.8%) compared to older (> 50 years) patients (1.5%), p= 0.019. Overall, 19 patients died (group A: 4.8%; B: 2.8%; C: 10%). In patients < 65 years all deaths (10) were leukemia-related, while in patients >65 years, all but one (8/9) deaths were leukemia-unrelated. The 6-year overall-survival was 94%, 97%, and 84% in pts 18-49 years, 50-64 years, and > 65 years of age (p=0.12)
Summary/Conclusion: Nilotinib as first-line treatment of newly diagnosed CP CML patients showed high rates of molecular responses in all age groups. However, compared to older patients, significant more progressions occurred in younger (18-49 years) ones; the causes of death in this group were all leukemia-related. In contrast, in elderly patients (>65 years) the causes of death were almost all leukemia-unrelated. ATEs were rare (2%) in patients of 18-49 years, while high rates (> 10%) of ATEs were recorded in those of 50-64 years; as expected, ATEs occurred even more frequently in patients > 65 years. These data suggest that while in patients > 50 years more attention should be focused on the prevention of ATEs, in younger ones more efforts are needed to avoid the progression of CML to accelerated/blast phase.
Gugliotta:Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Castagnetti:Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; ARIAD Pharmaceuticals: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Breccia:Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Ariad: Honoraria; Pfizer: Honoraria. Carella:Millenium: Speakers Bureau; Genentech: Speakers Bureau. Tiribelli:Ariad Pharmaceuticals: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Soverini:Ariad: Consultancy; Bristol-Myers Squibb: Consultancy; Novartis: Consultancy. Cavo:Janssen-Cilag: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Millennium: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Martinelli:Pfizer: Consultancy, Speakers Bureau; ARIAD: Consultancy; Roche: Consultancy; MSD: Consultancy; Novartis: Speakers Bureau; Amgen: Consultancy; Genentech: Consultancy; BMS: Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; ARIAD: Consultancy; Roche: Consultancy; MSD: Consultancy; Amgen: Consultancy; Genentech: Consultancy. Saglio:Ariad: Consultancy; Pfizer: Consultancy; Bristol Myers Squibb: Consultancy. Baccarani:Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Pfizer: Honoraria, Other: personal fees, Speakers Bureau; Ariad: Honoraria, Other: personal fees, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Rosti:Roche: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau; Incyte: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal