Abstract
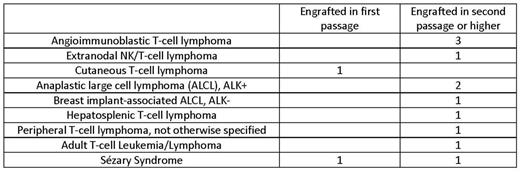
Lymphomas represent nearly 70 distinct diseases with unique clinical presentations, therapeutic responses and underlying biology. There is a pressing shortage of publically available cell line and in vivo models of nearly all of these diseases; T-cell lymphoma models are particularly under-represented compared to B-cell lymphomas, which has severely hampered efforts to understand and target their biology. The majority ofin vivo models of T-cell lymphomas are genetically-engineered mouse models, which often don't faithfully recapitulate human disease. To address this issue, we have established a repository of patient-derived xenografts (PDX) of lymphomas by engrafting human tumors into immunodeficient NOD/Scid/IL2rgnull mice with or without an MHC Class 1 deficiency (to prevent graft versus host disease). Blood and bone marrow specimens involved with tumor were injected by tail vein injection. Lymph node and extranodal biopsy specimens were implanted under the renal capsule as a 1x1x2mm tumor seed, which maintains the in situ microarchitecture. A description of T-cell lymphoma PDXs is included in the Table.
PDXs have been extensively characterized by immunohistochemistry (IHC), flow cytometry, transcriptome sequencing and targeted DNA sequencing. These studies have demonstrated retention of key architectural, cellular, and molecular features of the primary tumors. Flow cytometric analysis of patient tumors and their respective xenografts revealed highly concordant patterns of surface marker expression. IHC of murine tissues confirmed retention of tumor immunophenotypes, architecture, and even tissue tropism in the PDXs. For example, blood from a patient with Sézary Syndrome manifested in the skin of recipient mice when injected into the lateral tail vein. A breast implant-associated ALK-negative anaplastic large cell lymphoma (ALCL) implanted under the renal capsule metastasized to the liver and spleen while uniformly retaining CD30 positivity. A peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) specimen implanted under the renal capsule engrafted in the spleen, with the notable admixture of nonmalignant T cells and scattered EBV-positive B cells in first passage. T-cell receptor gene rearrangement PCR performed on this PTCL-NOS demonstrated an identical rearrangement pattern in the primary tumor and the PDX. An angioimmunoblastic T-cell lymphoma (AITL) specimen engrafted in spleen, lymph node and bone marrow within 6 weeks and serially transplanted through three generations in an orthotopic manner while maintaining a CD3+CD4+PD1+CD30partial immunophenotype.
The genetic characterization of the PDX models using a targeted DNA sequencing approach showed a mutational profile that clearly matched primary T-cell lymphoma samples and significantly expands the current repertoire of available pre-clinical models. For example, a PDX model of AITL showed mutations of TET2 and ARID1B; a model of an ALK-negative ALCL harbored mutations of STAT3 and STAT5. This massively extends the spectrum of clinically representative model systems that can be used to explore novel therapeutic strategies for T-cell lymphomas. Several early-passage PDXs have been used to generate T-cell lymphoma cells lines, including three cell lines from AITL PDX models. One of these AITL cell lines has proliferated through 30 passages and was validated by immunophenotype and molecular confirmation of bi-allelic TET2 mutations with loss of 6q, 7q, and 10q confirmed using Sanger and TruSeq Custom Amplicon Sequencings. To our knowledge, there have been no reports of an AITL cell line in the literature. Additional peripheral T-cell lymphoma cell lines are currently under development.
These lymphomas, along with a spectrum of PDXs of other hematologic malignancies, are available to collaborators through the online portal PRoXe (Public Repository of Xenografts) at www.proxe.org. These models represent a unique opportunity to interrogate biology and perform preclinical studies with in vivo models.
Jacobson:Kite: Membership on an entity's Board of Directors or advisory committees. Armand:Pfizer: Research Funding; Sequenta Inc: Research Funding; Merck: Consultancy, Research Funding; Roche: Research Funding; Infinity Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding. Shipp:Bristol-Myers Squibb: Consultancy, Research Funding; Cell Signaling: Honoraria; Merck, Gilead, Takeda: Other: Scientific Advisory Board; Bayer: Research Funding. Fisher:Pharmacyclics: Consultancy. Weinstock:Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal