Abstract
BACKGROUND: Mantle cell lymphoma (MCL) represents approximately 5-10% of all non-Hodgkin lymphoma (NHL) diagnosed annually in the US and Europe. In contrast to the slight male predominance of other non-Hodgkin lymphomas, most series report that approximately 80% of MCL patients are male. While the pathobiology underlying this gender-distribution imbalance is unknown, sex-related differences in androgen receptor (AR) expression in the hematopoietic system have been described, leading us to hypothesize that the gender imbalance in MCL may be mediated via differential sex-related effects on AR activity. Enzalutamide is a potent inhibitor of the AR axis, binding to the AR with high selectivity, and targeting multiple steps in AR signaling, including inhibition of AR ligand binding, AR nuclear translocation, and AR-mediated DNA binding.
OBJECTIVE: To evaluate AR expression in MCL cell lines and human specimens and explore the effect of AR-axis blockade with enzalutamide on MCL tumor cell proliferation.
METHODS: MCL cell lines (JVM-13, Maver-1, Jeko-1, Rec-1, JVM-2, Granta and Mino), non-MCL cell lines (SUDHL4, FL18 and Ramos) and prostate cancer cell lines (LNCaP, VCaP, PC3 and Du-145) were obtained from American Type Culture Collection (Rockville, Maryland, USA) and cultured in accordance with supplier recommended methods. Buffy coat specimens from three MCL patients with circulating tumor cells were utilized for RNA isolation and analysis of AR transcript expression. Additionally, formalin fixed samples from nine archival MCL tumor specimens were obtained for immunohistochemistry (IHC) analysis of AR staining. RNA was isolated and prepared for quantitative real time PCR (qRT-PCR) using primers for AR and PSA. All qRT-PCR experiments were performed in triplicate, and the housekeeping gene RPL13A was used as an endogenous control. Proliferation assays were carried out with cells brought up in serum free media, incubated under standard culture conditions for 24 hours, then plated in triplicate with the AR antagonist enzalutamide (10uM), the synthetic AR agonist R1881 (1nM), or the combination for 96 hours. Following the incubation period, cells were transferred to a flat-bottomed 96-well plate and proliferation was quantified using the CyQUANTTM Assay Kit.
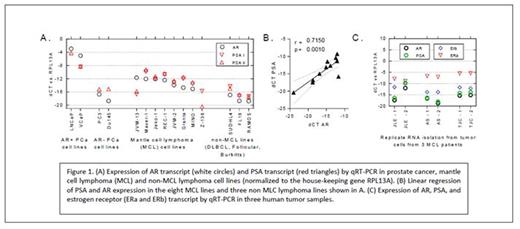
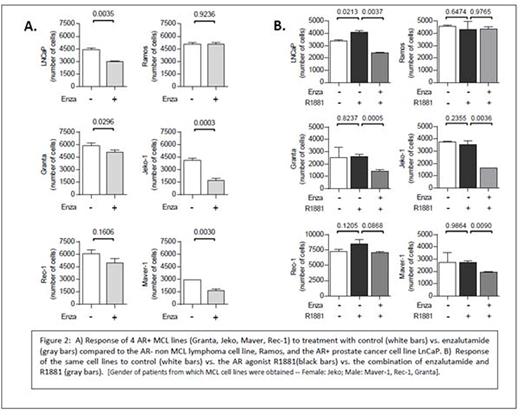
RESULTS: The median expression of AR transcript was significantly higher in MCL lines than in non-MCL B-NHL lines (normalized to the difference in cycle threshold (dCT) of the housekeeping gene RPL13A). The median dCT for AR was -12 in MCL lines (range -11 to -15) vs. -18 in non-MCL lines (p=0.006; Figure 1A), representing a 64 fold difference in transcript expression. Additionally, the tight correlation between the canonical AR-regulated target gene PSA and AR (r=.715, p=0.001; Figure 1B) suggests that AR expression within these MCL lines is functionally active and capable of driving transactivation of target genes. These findings were also observed in a limited number of frozen, patient-derived MCL tumors; where 2 of 3 samples demonstrated AR and PSA transcripts at levels similar to those observed in the MCL cell lines (Figure 1C). Staining of formalin fixed human MCL tumor cells for AR expression by IHC demonstrated a spectrum of expression; including tumors with no staining, tumors with predominantly cytoplasmic AR staining, and tumors with evidence of scattered nuclear AR staining. Proliferation assays of four different AR+ MCL lines (Granta, Jeko, Maver, Rec-1) with the AR agonist R1881, enzalutamide, or the combination demonstrated somewhat limited stimulation by agonist but consistent and statistically significant suppression of proliferation by enzalutamide (Figure 2). The magnitude of suppression in several of the MCL lines (eg, Granta and Jeko) was comparable to that observed in the AR+ LNCaP PCa cell line.
CONCLUSION: Functional AR transcript is overexpressed in both MCL cell lines and human tumor samples. Blockade of the AR-axis by enzalutamide effects consistent and reproducible suppression of MCL tumor growth, even in the presence of AR agonist R1881. These results have led to the conception and recent initiation of an NCCN funded phase II clinical trial evaluating the safety and clinical efficacy of enzalutamide as a novel therapy for patients with relapsed or refractory MCL (NCT02489123).
Gopal:Paid Consultancy- Gilead, Janssen, Seattle Genetics, Spectrum, Research funding- Gilead, Janssen, Pfizer, BMS, Merck, Teva, Takeda, Spectrum, Seattle Genetics: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal