Abstract
Background: T-cell prolymphocytic leukemia (T-PLL) is a rare and aggressive mature T-cell lymphoid leukemia. T-PLL is diagnosed based on characteristic immunophenotype, cytogenetic, and molecular aberrations (including members of the JAK-STAT signaling pathway) which may explain its aggressive clinical course. Cytogenetic features include chromosome (chr) 14 aberrations, accompanied by gene rearrangement involving proto-oncogenes TCL1 or MTCP1. In this analysis, we report our single-institution experience with T-PLL over the last 25 years.
Methods: We reviewed the clinico-pathologic records of 101 consecutive patients (pts) with T-PLL, who presented to our institution between 1990 and 2015. The survival (OS) of pts was calculated from the date of initial presentation to the date of last follow up. Kaplan-Meier product limit method was used to estimate the median OS.
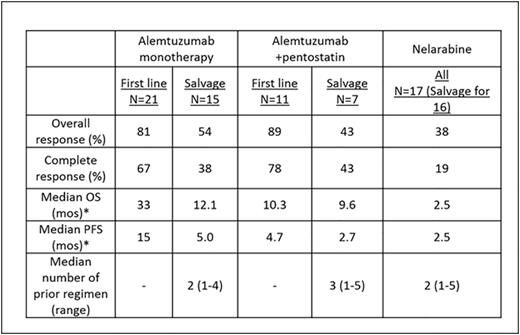
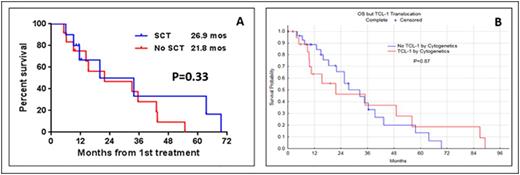
Results: Median age of pts was 64 years (range 35-87 years). Eighteen pts (18%) presented with a performance status of 2 or higher. Lymphadenopathy, splenomegaly, skin lesions, hepatomegaly and pleural effusion were seen in 58%, 37%, 30%, 8%, and 7% of pts, respectively. Complex karyotype and aberrations in chr 14 were seen in 54 (63%) and 42 (49%) pts, respectively. TCL1 expression by immunohistochemistry was performed in 29 pts. The overexpression of TCL1 was detected in 23/29 pts (79%). Among untreated pts, 18/47 (38%) had a chr abnormality involving TCL-1. At initial presentation to our center, 59 pts (58%) were untreated and 42 pts (42%) had relapsed/refractory disease with a median of 2 prior treatments (range 1-6). Seventy-one pts (70%) were treated at our institution. Median overall OS from diagnosis in all pts was 21.7 mos (Range 1.5-107.7 mos). Median OS was longer in untreated pts than in pts with relapsed/refractory disease (27 mos vs. 17 mos; P=0.08). In a multivariate analysis for assessing the prognostic factors for OS in 43 untreated pts in whom all variables were available, we found that the presence of a pleural effusion HR (95% CI) 5.21 (1-27; P <0.04), high absolute lymphocyte count 1.009 (1.002-1.016; P = 0.006) and complex karyotype 4.6 (1.2-16.9; P = 0.02) predicted for increased risk of death. Fifty four evaluable pts (32 untreated and 22 with relapsed disease) received treatment with alemtuzumab based regimen, 36 as monotherapy and 18 in combination with pentostatin. Seventeen evaluable pts (1 untreated and 16 with relapsed disease) were treated with nelarabine. Overall response rate (ORR), complete remission rate (CR), median OS and progression free survival (PFS) are summarized in Table-1. Among 39 previously untreated pts who received treatment at our institution, the distribution of treatment regimen was alemtuzumab-based (n=32), nelarabine (n=1), other nucleoside analogues (n=5) and combination chemotherapy (n=1). Twenty two pts (56%) achieved complete remission (CR) after frontline treatment. Ten pts underwent allogeneic stem cell transplantation (SCT) after achieving a remission after initial treatment with alemtuzumab either as a monotherapy or in combination with pentostatin. We did not observe any significant difference in OS (Figure-1A) or PFS among pts who did/did not undergo stem cell transplantation after achieving an initial remission with the frontline therapies. Furthermore, we observed that there was no significant difference in OS among pts with or without a chr abnormality involving TCL-1 after frontline therapy (Figure-1B).
Conclusions: In this retrospective analysis, we have observed that outcomes in T-PLL remain poor. We have shown that alemtuzumab has some therapeutic activity in pts with T-PLL but durable remissions are uncommon. Rarity of this disease limits the conduct of large scale clinical trials; hence multicenter collaborative effort is required to conduct prospective studies. In pts who achieve a CR, consolidation treatment with SCT did not improve survival in our series. Studies to further define the genomic imbalances in pts with T-PLL and identify potential therapeutic targets and pathways providing drug resistance in T-PLL are underway.
Summary of treatment response and outcomes according to the type of therapy
*Censored at stem cell transplant.
Summary of treatment response and outcomes according to the type of therapy
*Censored at stem cell transplant.
(A-B) - Survival of pts after treatment with frontline therapy A) with/without stem cell transplantation after initial remission. B) With/without TCL-1 rearrangements.
(A-B) - Survival of pts after treatment with frontline therapy A) with/without stem cell transplantation after initial remission. B) With/without TCL-1 rearrangements.
O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Wierda:Acerta: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Abbvie: Research Funding; Genentech: Research Funding. Jain:Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Abbvie: Research Funding; Servier: Consultancy, Honoraria; Seattle Genetics: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Infinity: Research Funding; Incyte: Research Funding; BMS: Research Funding. Thompson:Pharmacyclics: Consultancy, Honoraria. Kantarjian:BMS, Pfizer, Amgen, Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal