Abstract
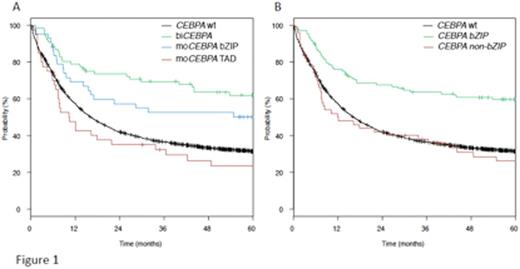
Mutations of the key myeloid transcription factor CCAAT/enhancer binding protein alpha (C/EBPa) are found in 5-10% of patients with acute myeloid leukemia (AML). Two mutational clusters exist, in the aminoterminal transcription activation domains (TAD1 or 2) and in the basic leucine zipper domain (bZIP) located at the carboxyterminal-part of the protein. Biallelic mutations (biCEBPA) have been found to be associated with improved outcome and are now included as an independent entity in the WHO-classification. In contrast, monoallelic CEBPA-mutations (moCEBPA) do not appear to provide prognostic information. We characterized a large cohort of AML patients for CEBPA mutations and further analyzed the mutational spectrum of mono- and biallelic CEBPA-mutant AML patients to better understand potential differences in the biology of these groups. Patients and Methods: Patients (including all age groups) analyzed had a newly diagnosed AML and were registered in clinical protocols of the Study Alliance Leukemia (SAL)(AML96, AML2003 or AML60+, SORAML) or the SAL-register. Screening for CEBPA mutations was done using PCR and capillary electrophoresis. All identified CEBPA mutations were confirmed using conventional Sanger sequencing and the samples were further analyzed using next generation sequencing (Trusight Myeloid Panel, Illumina) for the presence of associated alterations. Results: In the 4578 patients analyzed, 228 (5%) with CEBPA-mutations were identified. An initial analysis revealed substantial clinical differences between the different mutation subtypes. Patients with biCEBPA (n=111) were significantly younger (median age 46 yrs) than wt-CEBPA patients (median 57 yrs; p<.001). Interestingly, single bZIP mutant patients (n=64) had a similar median age (50 yrs.) as biCEBPA, whereas single TAD mutant patients (n=53) were significantly older (median 63 yrs.). In addition, WBC counts, CD34 positivity as well as the history of prior MDS differed between the subgroups (single TAD mutant had significantly lower WBC counts, lower rate of CD34 positivity and had a higher rate of prior MDS than biCEBPA and single bZIP mutant patients). Along with this, the distribution of co-mutations differed significantly between the subgroups, especially GATA2 mutations were more common in biCEBPA and single bZIP mutant patients (37% and 34%, respectively) compared to only 3% (single TAD)(p=.001). A similar pattern was seen for mutations in DNMT3A (8% biCEBPA, 20% single bZIP vs. 36% single TAD; p=.001), and NPM1 (3% biCEBPA, 8% single bZIP, 32% single TAD; p<.001). In 2897 patients, the different CEBPA mutations were correlated with outcome. This analysis indicated a differential effect of the individual mutations on outcome, with an improved rate of complete remission (CR), overall survival (OS) and event free survival (EFS) for biCEBPA and single bZIP mutations in univariate and multivariate analyses (shown for OS in Figure 1a). Given the similarity of single bZIP and biCEBPA mutations, it appears reasonable to speculate on a common mechanistical background, since most of the biCEBPA mutants include a bZIP alteration. Recent experimental evidence generated by several groups indeed supports a specific role of these bZIP missense mutations. To address this in the clinical context, we regrouped patients with mutant CEBPA into patients with (n=157) or without bZIP mutations (n=71), irrespective of the biallelic status. As illustrated in Figure 1b, the bZIP mutant group had a significantly better OS, similar results were obtained for EFS and CR. In multivariate analysis, the presence of a bZIP mutation was the strongest indicator for achievement of CR (HR 7.5, 95% CI: 3-19; p<.001), and together with favorable cytogenetics the factor associated with best OS (HR: .48; 95% CI .36-.64; p<.001). In conclusion, our results obtained in one of the largest cohorts of AML patients analyzed for CEBPA mutations indicate that especially the presence of a missense bZIP mutation is associated with a favorable outcome in AML patients. These data point to substantial differences in prognostic implications of individual CEBPA mutations and support the major functional divergence of these alterations. If confirmed, these results might necessitate further refinement of their use in AML-classification.
Middeke:Sanofi: Honoraria. Platzbecker:Janssen-Cilag: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; TEVA Pharmaceutical Industries: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Thiede:AgenDix: Employment, Other: Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal