Abstract
Introduction: Immune checkpoint inhibitors (ICIs) have shown significant efficacy in solid malignancies and recently in Hodgkin's disease. However, there are presently no biomarkers that predict which cancer types and/or individual patients will benefit from ICIs. Despite intensification of chemotherapeutic regimens, survival rates in acute myeloid leukemia (AML) have plateaued. We are therefore, performing studies to characterize the immune phenotype and genetic profile of AML patients who may respond to ICI therapy. Specifically, we are identifying cell subsets present in the marrow of patients with AML, evaluating their differentiation and activation status, and quantifying the density of immune modifiers. We are also performing functional assays to define the status of T cells in the AML microenvironment by evaluating the responsiveness of these cells to stimulation. Additionally, these studies assess the impact of several ICIs on the proliferative potential and cytokine production capacity of T cells resident in the bone marrow of AML patients.
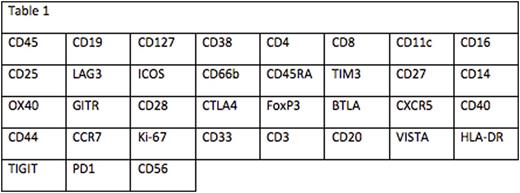
Methods: Immunophenotyping was performed on the diagnostic bone marrow (Dx BM), diagnostic peripheral blood (Dx PB), and remission bone marrow (CR BM) for a single patient with AML. These studies used a time-of-flight mass cytometer (CyTOF) by staining samples with a panel containing 35 antibodies to surface markers and cytokines (Table 1). T cells were defined and then differentiated based on the presence of CD3 and CD4 or CD8. T cells were further classified into naive, central memory (CM), effector memory (EM), and effector cells. Functional assays measured proliferative capacity and cytokine production profile of bone marrow T cells in response to CD3 stimulation for the Dx BM and Dx PB. Changes to the baseline T cell functional profile in the presence of antibodies against PD1, CTLA4, TIM3, and TIGIT were measured in the Dx BM.
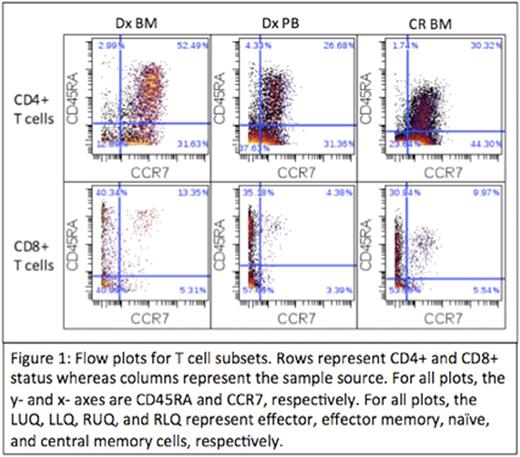
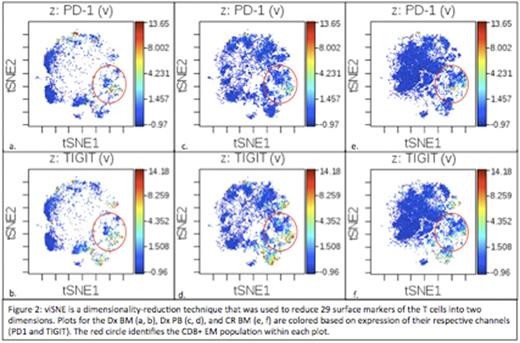
Results: T cell percentages were 3.14%, 5.46%, and 17.1% for the Dx BM, Dx, PB, and CR BM, respectively. CD4:CD8 ratios were 3.5, 2.9, and 4.5, respectively. EM cells made up the largest percentage of CD8+ T cells in the Dx BM (40.99%), Dx PB (57.06%), and CR BM (53.55%). Naïve (52.49%), EM (37.63%) and CM (44.3%) cells made up the largest percentages of CD4+ T cells in the Dx BM, Dx PB, and CR BM, respectively (Figure 1). Checkpoint expression was absent (median intensity < 1) on all T cells except for some CD8+ subsets (Figure 2). Within the Dx BM, the effector (1.93) and EM (4.34) cells expressed TIGIT and the EM cells expressed PD1 (1.92). Within the Dx PB, the effector (1.81) and EM (4.0) cells expressed TIGIT. Within the CR BM, the effector cells (2.3) expressed TIGIT. In response to CD3 stimulation, 57.5% of the Dx PB T cells divided while there was no proliferation observed in the Dx BM. In response to CD3 stimulation and PD1 blockade, 28.2% of the Dx BM T cells divided and there was a 4-fold increase in IFNγ production. There was no division in the Dx BM T cells in response to TIGIT blockade but there was a 10-fold and 121-fold increase in IL-2 and IFNγ production, respectively. There was no significant proliferation or cytokine production in response to TIM3 or CTLA4 blockade.
Conclusion: We observed differential T cell responses to stimulation in the presence of ICIs in this patient. The Dx PB T cells were capable of proliferating in the presence of CD3 stimulation alone whereas the Dx BM T cells were only able to divide in the presence of PD1 blocking antibody. PD1 blockade elicited proliferation and low levels of IFNγ production whereas TIGIT blockade elicited negligible proliferation but high levels of IFNγ production. There was low but differential checkpoint expression on T cells, with PD1 and TIGIT having the highest expression, specifically on CD8+ T cell subsets. The predominant CD8+ T cell subset, along with differential checkpoint expression, persisted into the CR BM but there was a shift in the predominant CD4+ T cell subset. These data suggest the presence of immune suppression in the bone marrow microenvironment that may be amenable to checkpoint inhibition.
Huang:Janssen Research & Development, LLC: Employment, Equity Ownership. Sasser:Janssen Research & Development, LLC: Employment. Adams:Janssen Research & Development, LLC: Employment. Druker:Agios: Honoraria; Ambit BioSciences: Consultancy; ARIAD: Patents & Royalties, Research Funding; Array: Patents & Royalties; AstraZeneca: Consultancy; Blueprint Medicines: Consultancy, Equity Ownership, Other: travel, accommodations, expenses ; BMS: Research Funding; CTI: Equity Ownership; Curis: Patents & Royalties; Cylene: Consultancy, Equity Ownership; D3 Oncology Solutions: Consultancy; Gilead Sciences: Consultancy, Other: travel, accommodations, expenses ; Lorus: Consultancy, Equity Ownership; MolecularMD: Consultancy, Equity Ownership, Patents & Royalties; Novartis: Research Funding; Oncotide Pharmaceuticals: Research Funding; Pfizer: Patents & Royalties; Roche: Consultancy. Lind:Janssen: Research Funding; Fluidigm: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal