Abstract
With a median age of 67 years, a significant proportion of patients with acute myeloid leukaemia (AML) are not considered suitable for conventional induction chemotherapy with "3+7" or related regimens. Such patients are typically treated with either demethylation agents or low-dose ara-C (LDAC). While demethylation agents appear to extend median survival, this does not convert into a greater long-term survival percentage, and 2 year survival is typically of the order of 10%. There is clearly a need to identify treatments which can offer the prospect of better long-term overall survival (OS).
Heat shock protein 90 (HSP90) acts as a molecular chaperone in a diverse range of cellular functions, including kinase dependant signalling and chromatin/epigenetic remodelling. Overexpression of HSP90 is reported in AML and correlates with a worse outcome (Thomas etal Leuk Res 2005).Ganetespib is a novel triazolone heterocyclic compound with significant activity in downregulating HSP90 client protein levels. In preclinical testing ganetespib appeared to show promise when compared with LDAC (Lazenby etal Leuk Res 2015) and it was therefore included, combined with LDAC, as part of the LI-1 "pick-a-winner" trial. The "pick-a-winner" design assesses several treatments simultaneously in a randomised fashion, in this case versus LDAC. There are two interim assessment points. At the first, following the accrual of 50 patients to each arm of the comparison, remission rates must be increased by at least 2.5%; at the second, after approximately 170 deaths are observed, the hazard ratio in favour of the novel therapy must be less than 0.85. The ultimate aim is to double 2-year OS from 11% to 22%, equivalent to a hazard ratio of 0.70.
Ganetespib was given at 120mg/m2 as a 1 hour intravenous infusion on days 1, 8, 15, 22 and 29 of each cycle of treatment. LDAC was given at 20mg bd subcutaneously on days 1-10. To access the ganetespib randomisation, patients had to fulfil specific cardiac entry criteria. Toxicities were defined as NCICTC v3.
The study was closed prematurely after 218 patients had been accrued, because the drug company could no longer support ganetespib. Analyses shown here are presented based upon 162 events and a median follow-up of 24.8 months, and are therefore indicative of the decision that would have been taken at the second interim analysis had the trial continued.
Results: Between 10/2012 and 3/2016, a total of 218 patients, median age 75.5 years (range 61-90) were recruited to the study. Overall 60% were male; 66% had de novo AML, 25% secondary AML, and 10% high risk MDS; 1% had favourable, 78% intermediate and 21% adverse cytogenetics. By validated Wheatley index, 1% were good risk, 43% standard risk and 56% poor risk. The median number of courses delivered was 2 (range 0-8) in either arm of the trial.
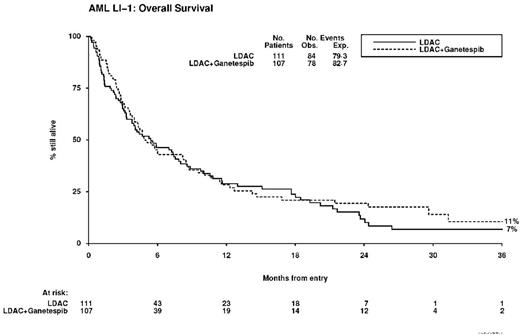
Overall, a complete marrow response was seen in 17% of patients (ganetespib 18%; LDAC 16%, OR 0.85 (0.41-1.73) p=0.6). There was no increase in 30-day mortality with ganetespib (10% vs 14%, HR 0.65 (0.29-1.42) p=0.3), but OS was not significantly improved (2 year OS 19% vs 12%, HR 0.89 (0.65-1.21) p=0.5). There was no evidence of any difference in relapse-free survival (HR 0.97 (0.42-2.25) p=0.9). There was significantly greater toxicity seen with ganetespib in course 1, leading to significantly more inpatient days (median 11 vs 5, p=0.04). Causes of death were (LDAC+Ganetespib vs LDAC: resistant/recurrent disease 60 vs 60; infection 8 vs 14; haemorrhage 4 vs 3; pulmonary 1 vs 0; multiple 4 vs 5; other/unknown 1 vs 2).
Conclusion: Despite promising data in vitro and acceptable tolerability we did not find evidence for ganetespib to deliver a significant survival benefit. On preliminary data after 162 events, the treatment effect is less than that required by the second continuation criterion for the study.
Acknowledgments: We are grateful to Synta for providing drug and support for this Investigator Initiated Study.
Hills:TEVA: Honoraria. Copland:Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Ariad: Honoraria; Amgen: Honoraria; Shire: Honoraria. Burnett:CTI Life Sciences, London: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal